Label-Free Saliva Test for Rapid Detection of Coronavirus Using Nanosensor-Enabled SERS
- PMID: 36978782
- PMCID: PMC10045265
- DOI: 10.3390/bioengineering10030391
Label-Free Saliva Test for Rapid Detection of Coronavirus Using Nanosensor-Enabled SERS
Abstract
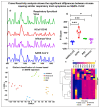
The recent COVID-19 pandemic has highlighted the inadequacies of existing diagnostic techniques and the need for rapid and accurate diagnostic systems. Although molecular tests such as RT-PCR are the gold standard, they cannot be employed as point-of-care testing systems. Hence, a rapid, noninvasive diagnostic technique such as Surface-enhanced Raman scattering (SERS) is a promising analytical technique for rapid molecular or viral diagnosis. Here, we have designed a SERS- based test to rapidly diagnose SARS-CoV-2 from saliva. Physical methods synthesized the nanostructured sensor. It significantly increased the detection specificity and sensitivity by ~ten copies/mL of viral RNA (~femtomolar concentration of nucleic acids). Our technique combines the multiplexing capability of SERS with the sensitivity of novel nanostructures to detect whole virus particles and infection-associated antibodies. We have demonstrated the feasibility of the test with saliva samples from individuals who tested positive for SARS-CoV-2 with a specificity of 95%. The SERS-based test provides a promising breakthrough in detecting potential mutations that may come up with time while also preparing the world to deal with other pandemics in the future with rapid response and very accurate results.
Keywords: COVID-19; SERS; coronavirus; nanosensors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Orooji Y., Sohrabi H., Hemmat N., Oroojalian F., Baradaran B., Mokhtarzadeh A., Mohaghegh M., Karimi-Maleh H. An Overview on SARS-CoV-2 (COVID-19) and Other Human Coronaviruses and Their Detection Capability via Amplification Assay, Chemical Sensing, Biosensing, Immunosensing, and Clinical Assays. Nano-Micro Lett. 2020;13:18. doi: 10.1007/s40820-020-00533-y. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

