Noninvasive Methods to Detect Reactive Oxygen Species as a Proxy of Seed Quality
- PMID: 36978875
- PMCID: PMC10045522
- DOI: 10.3390/antiox12030626
Noninvasive Methods to Detect Reactive Oxygen Species as a Proxy of Seed Quality
Abstract
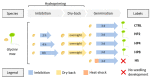
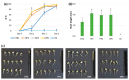
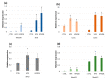
ROS homeostasis is crucial to maintain radical levels in a dynamic equilibrium within physiological ranges. Therefore, ROS quantification in seeds with different germination performance may represent a useful tool to predict the efficiency of common methods to enhance seed vigor, such as priming treatments, which are still largely empirical. In the present study, ROS levels were investigated in an experimental system composed of hydroprimed and heat-shocked seeds, thus comparing materials with improved or damaged germination potential. A preliminary phenotypic analysis of germination parameters and seedling growth allowed the selection of the best-per-forming priming protocols for species like soybean, tomato, and wheat, having relevant agroeconomic value. ROS levels were quantified by using two noninvasive assays, namely dichloro-dihydro-fluorescein diacetate (DCFH-DA) and ferrous oxidation-xylenol orange (FOX-1). qRT-PCR was used to assess the expression of genes encoding enzymes involved in ROS production (respiratory burst oxidase homolog family, RBOH) and scavenging (catalase, superoxide dismutase, and peroxidases). The correlation analyses between ROS levels and gene expression data suggest a possible use of these indicators as noninvasive approaches to evaluate seed quality. These findings are relevant given the centrality of seed quality for crop production and the potential of seed priming in sustainable agricultural practices.
Keywords: DCFH-DA; FOX-1; Glycine max; ROS; gene expression; heat-shock; seed priming; seed quality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Milivojević M., Ripka Z., Petrović T. ISTA rules changes in seed germination testing at the beginning of the 21st century. J. Process Energy Agric. 2018;22:40–45. doi: 10.5937/JPEA1801040M. - DOI
-
- Huang M., Wang Q.G., Zhu Q.B., Qin J.W., Huang G. Review of seed quality and safety tests using optical sensing technologies. Seed Sci. Technol. 2015;43:337–366. doi: 10.15258/sst.2015.43.3.16. - DOI
-
- Rahman A., Cho B.K. Assessment of seed quality using non-destructive measurement techniques: A review. Seed Sci. Res. 2016;26:285–305. doi: 10.1017/S0960258516000234. - DOI
-
- Pagano A., Forti C., Gualtieri C., Balestrazzi A., Macovei A. Oxidative stress and antioxidant defense in germinating seeds. A Q&A session. In: Hasanuzzaman M., Fotopoulos V., Nahar K., Fujita M., editors. Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms. John Wiley & Sons Ltd.; Hoboken, NJ, USA: 2019. pp. 267–289.
LinkOut - more resources
Full Text Sources

