The Transcription Factor NRF2 Has Epigenetic Regulatory Functions Modulating HDACs, DNMTs, and miRNA Biogenesis
- PMID: 36978889
- PMCID: PMC10045347
- DOI: 10.3390/antiox12030641
The Transcription Factor NRF2 Has Epigenetic Regulatory Functions Modulating HDACs, DNMTs, and miRNA Biogenesis
Abstract
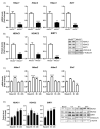
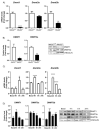
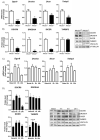
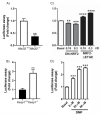
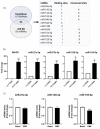
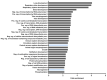
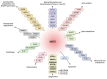
The epigenetic regulation of gene expression is a complex and tightly regulated process that defines cellular identity and is associated with health and disease processes. Oxidative stress is capable of inducing epigenetic modifications. The transcription factor NRF2 (nuclear factor erythroid-derived 2-like 2) is a master regulator of cellular homeostasis, regulating genes bearing antioxidant response elements (AREs) in their promoters. Here, we report the identification of ARE sequences in the promoter regions of genes encoding several epigenetic regulatory factors, such as histone deacetylases (HDACs), DNA methyltransferases (DNMTs), and proteins involved in microRNA biogenesis. In this research, we study this possibility by integrating bioinformatic, genetic, pharmacological, and molecular approaches. We found ARE sequences in the promoter regions of genes encoding several HDACs, DNMTs, and proteins involved in miRNA biogenesis. We confirmed that NRF2 regulates the production of these genes by studying NRF2-deficient cells and cells treated with dimethyl fumarate (DMF), an inducer of the NRF2 signaling pathway. In addition, we found that NRF2 could be involved in the target RNA-dependent microRNA degradation (TDMD) of miR-155-5p through its interaction with Nfe2l2 mRNA. Our data indicate that NRF2 has an epigenetic regulatory function, complementing its traditional function and expanding the regulatory dimensions that should be considered when developing NRF2-centered therapeutic strategies.
Keywords: DMF; DNMT; HDAC; NRF2; TDMD; epigenetics; miRNA; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Epigenetic Regulation of NRF2/KEAP1 by Phytochemicals.Antioxidants (Basel). 2020 Sep 14;9(9):865. doi: 10.3390/antiox9090865. Antioxidants (Basel). 2020. PMID: 32938017 Free PMC article. Review.
-
Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P + cells through epigenetics modifications.AAPS J. 2014 Jul;16(4):727-35. doi: 10.1208/s12248-014-9613-8. Epub 2014 May 16. AAPS J. 2014. PMID: 24830944 Free PMC article.
-
Genistein Induces Alterations of Epigenetic Modulatory Signatures in Human Cervical Cancer Cells.Anticancer Agents Med Chem. 2018;18(3):412-421. doi: 10.2174/1871520617666170918142114. Anticancer Agents Med Chem. 2018. PMID: 28925878
-
The dietary flavone luteolin epigenetically activates the Nrf2 pathway and blocks cell transformation in human colorectal cancer HCT116 cells.J Cell Biochem. 2018 Nov;119(11):9573-9582. doi: 10.1002/jcb.27275. Epub 2018 Aug 20. J Cell Biochem. 2018. PMID: 30129150 Free PMC article.
-
Targeting epigenetic and post-translational modifications of NRF2: key regulatory factors in disease treatment.Cell Death Discov. 2025 Apr 21;11(1):189. doi: 10.1038/s41420-025-02491-z. Cell Death Discov. 2025. PMID: 40258841 Free PMC article. Review.
Cited by
-
The Roles of NFR2-Regulated Oxidative Stress and Mitochondrial Quality Control in Chronic Liver Diseases.Antioxidants (Basel). 2023 Oct 29;12(11):1928. doi: 10.3390/antiox12111928. Antioxidants (Basel). 2023. PMID: 38001781 Free PMC article. Review.
-
RIPK1 expression and inhibition in tauopathies: implications for neuroinflammation and neuroprotection.Front Neurosci. 2025 Jan 27;18:1530809. doi: 10.3389/fnins.2024.1530809. eCollection 2024. Front Neurosci. 2025. PMID: 39931431 Free PMC article.
-
Decoding pain chronification: mechanisms of the acute-to-chronic transition.Front Mol Neurosci. 2025 Jun 26;18:1596367. doi: 10.3389/fnmol.2025.1596367. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40642387 Free PMC article. Review.
-
Synergistic Antioxidant Effects of C3G-Enriched Oryza sativa L. cv. RD83 Extract and α-Tocopherol Against H2O2-Induced Oxidative Stress in SH-SY5Y Cells.Int J Mol Sci. 2025 Jul 5;26(13):6490. doi: 10.3390/ijms26136490. Int J Mol Sci. 2025. PMID: 40650264 Free PMC article.
-
Pterostilbene Reverses Epigenetic Silencing of Nrf2 and Enhances Antioxidant Response in Endothelial Cells in Hyperglycemic Microenvironment.Nutrients. 2024 Jun 27;16(13):2045. doi: 10.3390/nu16132045. Nutrients. 2024. PMID: 38999793 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

