Different Effects and Mechanisms of Selenium Compounds in Improving Pathology in Alzheimer's Disease
- PMID: 36978950
- PMCID: PMC10045564
- DOI: 10.3390/antiox12030702
Different Effects and Mechanisms of Selenium Compounds in Improving Pathology in Alzheimer's Disease
Abstract
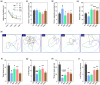
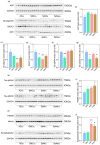
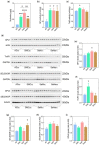
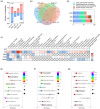
Owing to the strong antioxidant capacity of selenium (Se) in vivo, a variety of Se compounds have been shown to have great potential for improving the main pathologies and cognitive impairment in Alzheimer's disease (AD) models. However, the differences in the anti-AD effects and mechanisms of different Se compounds are still unclear. Theoretically, the absorption and metabolism of different forms of Se in the body vary, which directly determines the diversification of downstream regulatory pathways. In this study, low doses of Se-methylselenocysteine (SMC), selenomethionine (SeM), or sodium selenate (SeNa) were administered to triple transgenic AD (3× Tg-AD) mice for short time periods. AD pathology, activities of selenoenzymes, and metabolic profiles in the brain were studied to explore the similarities and differences in the anti-AD effects and mechanisms of the three Se compounds. We found that all of these Se compounds significantly increased Se levels and antioxidant capacity, regulated amino acid metabolism, and ameliorated synaptic deficits, thus improving the cognitive capacity of AD mice. Importantly, SMC preferentially increased the expression and activity of thioredoxin reductase and reduced tau phosphorylation by inhibiting glycogen synthase kinase-3 beta (GSK-3β) activity. Glutathione peroxidase 1 (GPx1), the selenoenzyme most affected by SeM, decreased amyloid beta production and improved mitochondrial function. SeNa improved methionine sulfoxide reductase B1 (MsrB1) expression, reflected in AD pathology as promoting the expression of synaptic proteins and restoring synaptic deficits. Herein, we reveal the differences and mechanisms by which different Se compounds improve multiple pathologies of AD and provide novel insights into the targeted administration of Se-containing drugs in the treatment of AD.
Keywords: Alzheimer’s disease; Se-methylselenocysteine; metabolism; selenomethionine; sodium selenate; synaptic deficits.
Conflict of interest statement
There are no conflict of interest to declare.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

