Nitric Oxide/Nitric Oxide Synthase System in the Pathogenesis of Neurodegenerative Disorders-An Overview
- PMID: 36979000
- PMCID: PMC10045816
- DOI: 10.3390/antiox12030753
Nitric Oxide/Nitric Oxide Synthase System in the Pathogenesis of Neurodegenerative Disorders-An Overview
Abstract
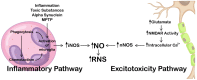
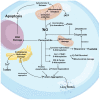
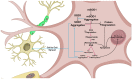
Nitric oxide, a ubiquitous molecule found throughout the natural world, is a key molecule implicated in many central and benefic molecular pathways and has a well-established role in the function of the central nervous system, as numerous studies have previously shown. Dysregulation of its metabolism, mainly the upregulation of nitric oxide production, has been proposed as a trigger and/or aggravator for many neurological affections. Increasing evidence supports the implication of this molecule in prevalent neurodegenerative diseases, such as Parkinson's disease, Alzheimer's disease, or amyotrophic lateral sclerosis. The mechanisms proposed for its neurotoxicity mainly center around the increased quantities of nitric oxide that are produced in the brain, their cause, and, most importantly, the pathological metabolic cascades created. These cascades lead to the formation of neuronal toxic substances that impair the neurons' function and structure on multiple levels. The purpose of this review is to present the main causes of increased pathological production, as well as the most important pathophysiological mechanisms triggered by nitric oxide, mechanisms that could help explain a part of the complex picture of neurodegenerative diseases and help develop targeted therapies.
Keywords: Alzheimer’s disease; Parkinson’s disease; amyotrophic lateral sclerosis; neurodegenerative disorders; nitric oxide; nitric oxide synthase; superoxide dismutase 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Role of Nitric Oxide Synthase in Normal Brain Function and Pathophysiology of Neural Diseases | IntechOpen. [(accessed on 31 October 2022)]. Available online: https://www.intechopen.com/chapters/53963.
Publication types
LinkOut - more resources
Full Text Sources

