Exploring the Long-Term Tissue Accumulation and Excretion of 3 nm Cerium Oxide Nanoparticles after Single Dose Administration
- PMID: 36979013
- PMCID: PMC10045098
- DOI: 10.3390/antiox12030765
Exploring the Long-Term Tissue Accumulation and Excretion of 3 nm Cerium Oxide Nanoparticles after Single Dose Administration
Abstract
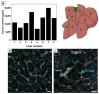
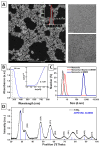
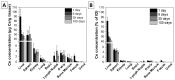
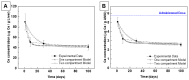
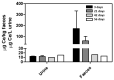
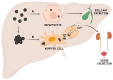
Nanoparticle (NP) pharmacokinetics significantly differ from traditional small molecule principles. From this emerges the need to create new tools and concepts to harness their full potential and avoid unnecessary risks. Nanoparticle pharmacokinetics strongly depend on size, shape, surface functionalisation, and aggregation state, influencing their biodistribution, accumulation, transformations, and excretion profile, and hence their efficacy and safety. Today, while NP biodistribution and nanoceria biodistribution have been studied often at short times, their long-term accumulation and excretion have rarely been studied. In this work, 3 nm nanoceria at 5.7 mg/kg of body weight was intravenously administrated in a single dose to healthy mice. Biodistribution was measured in the liver, spleen, kidney, lung, brain, lymph nodes, ovary, bone marrow, urine, and faeces at different time points (1, 9, 30, and 100 days). Biodistribution and urinary and faecal excretion were also studied in rats placed in metabolic cages at shorter times. The similarity of results of different NPs in different models is shown as the heterogeneous nanoceria distribution in organs. After the expectable accumulation in the liver and spleen, the concentration of cerium decays exponentially, accounting for about a 50% excretion of cerium from the body in 100 days. Cerium ions, coming from NP dissolution, are most likely excreted via the urinary tract, and ceria nanoparticles accumulated in the liver are most likely excreted via the hepatobiliary route. In addition, nanoceria looks safe and does not damage the target organs. No weight loss or apathy was observed during the course of the experiments.
Keywords: NP dissolution; NP excretion; NP long-term accumulation; nanoceria; nanoparticle biodistribution; nanopharmacokinetics; nanosafety.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lin J., Huang Z., Liu J., Huang Z., Liu Y., Liu Q., Yang Z., Li R., Wu X., Shi Z., et al. Neuroprotective Effect of Ketone Metabolism on Inhibiting Inflammatory Response by Regulating Macrophage Polarization after Acute Cervical Spinal Cord Injury in Rats. Front. Neurosci. 2020;14:583611. doi: 10.3389/fnins.2020.583611. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

