Gene Editing of the Follicle-Stimulating Hormone Gene to Sterilize Channel Catfish, Ictalurus punctatus, Using a Modified Transcription Activator-like Effector Nuclease Technology with Electroporation
- PMID: 36979084
- PMCID: PMC10044888
- DOI: 10.3390/biology12030392
Gene Editing of the Follicle-Stimulating Hormone Gene to Sterilize Channel Catfish, Ictalurus punctatus, Using a Modified Transcription Activator-like Effector Nuclease Technology with Electroporation
Abstract
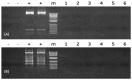
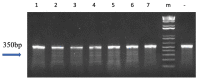
Follicle-stimulating hormone (fsh) plays an important role in sexual maturation in catfish. Knocking out the fsh gene in the fish zygote should suppress the reproduction of channel catfish (Ictalurus punctatus). In this study, transcription activator-like effector nuclease (TALEN) plasmids targeting the fsh gene were electroporated into fertilized eggs with the standard double electroporation technique. Targeted fsh cleavage efficiency was 63.2% in P1fsh-knockout catfish. Ten of fifteen (66.7%) control pairs spawned, and their eggs had 32.3-74.3% average hatch rates in 2016 and 2017. Without hormone therapy, the spawning rates of P1 mutants ranged from 33.3 to 40.0%, with an average egg hatching rate of 0.75%. After confirmation of the low fertility of P1 mutants in 2016, human chorionic gonadotropin (HCG) hormone therapy improved the spawning rates by 80% for female mutants and 88.9% for male mutants, and the mean hatch rate was 35.0% for F1 embryos, similar to that of the controls (p > 0.05). Polymerase chain reaction (PCR) identification showed no potential TALEN plasmid integration into the P1 channel catfish genome. Neither the P1 nor the F1 mutant fish showed any noticeable changes in in body weight, survival rate, and hatching rate when the reproductive gene was knocked out. F1 families had a mean inheritance rate of 50.3%. The results brought us one step closer to allowing implementation of certain genetic techniques to aquaculture and fisheries management, while essentially eliminating the potential environment risk posed by transgenic, hybrid, and exotic fish as well as domestic fish.
Keywords: channel catfish; follicle-stimulating hormone; hormone therapy; transcription activator-like effector nucleases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Dunham R.A., Ramboux A.C., Duncan P.L., Hayat M., Chen T.T., Lin C.M., Kight K., Gonzalez-Villasenor I., Powers D.A. Transfer, Expression, and Inheritance of Salmonid Growth Hormone Genes in Channel Catfish, Ictalurus punctatus, and Effects on Performance Traits. Mol. Mar. Biol. Biotechnol. 1992;1:380–389. - PubMed
-
- Hedrick P.W. Invasion of Transgenes from Salmon or Other Genetically Modified Organisms into Natural Populations. Can. J. Fish. Aquat. Sci. 2001;58:2317. doi: 10.1139/f01-161. - DOI
-
- Patiño R., Thomas P. Effects of Gonadotropin on Ovarian Intrafollicular Processes during the Development of Oocyte Maturational Competence in a Teleost, the Atlantic Croaker: Evidence for Two Distinct Stages of Gonadotropic Control of Final Oocyte Maturation. Biol. Reprod. 1990;43:818–827. doi: 10.1095/biolreprod43.5.818. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

