CAR-T Cells in the Treatment of Ovarian Cancer: A Promising Cell Therapy
- PMID: 36979400
- PMCID: PMC10046142
- DOI: 10.3390/biom13030465
CAR-T Cells in the Treatment of Ovarian Cancer: A Promising Cell Therapy
Abstract
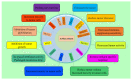
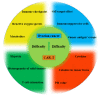
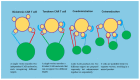
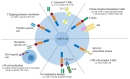
Ovarian cancer (OC) is among the most common gynecologic malignancies with a poor prognosis and a high mortality rate. Most patients are diagnosed at an advanced stage (stage III or IV), with 5-year survival rates ranging from 25% to 47% worldwide. Surgical resection and first-line chemotherapy are the main treatment modalities for OC. However, patients usually relapse within a few years of initial treatment due to resistance to chemotherapy. Cell-based therapies, particularly adoptive T-cell therapy and chimeric antigen receptor T (CAR-T) cell therapy, represent an alternative immunotherapy approach with great potential for hematologic malignancies. However, the use of CAR-T-cell therapy for the treatment of OC is still associated with several difficulties. In this review, we comprehensively discuss recent innovations in CAR-T-cell engineering to improve clinical efficacy, as well as strategies to overcome the limitations of CAR-T-cell therapy in OC.
Keywords: CAR; CAR-T; immunotherapy; ovarian cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- International Agency for Research on Cancer Survival, Incidence, and Mortality over Time. [(accessed on 23 December 2022)]. Available online: https://gco.iarc.fr/survival/survmark/
-
- Majzner R.G., Mackall C.L. Tumor Antigen Escape from CAR T-cell Therapy. Cancer Discov. 2018;8:1219–1226. doi: 10.1158/2159-8290.CD-18-0442. - DOI - PubMed
-
- Dudley M.E., Wunderlich J.R., Robbins P.F., Yang J.C., Hwu P., Schwartzentruber D.J., Topalian S.L., Sherry R., Restifo N.P., Hubicki A.M., et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

