Low-Affinity/High-Selectivity Dopamine Transport Inhibition Sufficient to Rescue Cognitive Functions in the Aging Rat
- PMID: 36979402
- PMCID: PMC10046369
- DOI: 10.3390/biom13030467
Low-Affinity/High-Selectivity Dopamine Transport Inhibition Sufficient to Rescue Cognitive Functions in the Aging Rat
Abstract
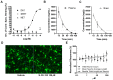
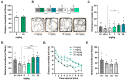
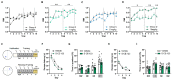
The worldwide increase in cognitive decline, both in aging and with psychiatric disorders, warrants a search for pharmacological treatment. Although dopaminergic treatment approaches represent a major step forward, current dopamine transporter (DAT) inhibitors are not sufficiently specific as they also target other transporters and receptors, thus showing unwanted side effects. Herein, we describe an enantiomerically pure, highly specific DAT inhibitor, S-CE-123, synthetized in our laboratory. Following binding studies to DAT, NET and SERT, GPCR and kinome screening, pharmacokinetics and a basic neurotoxic screen, S-CE-123 was tested for its potential to enhance and/or rescue cognitive functions in young and in aged rats in the non-invasive reward-motivated paradigm of a hole-board test for spatial learning. In addition, an open field study with young rats was carried out. We demonstrated that S-CE-123 is a low-affinity but highly selective dopamine reuptake inhibitor with good bioavailability. S-CE-123 did not induce hyperlocomotion or anxiogenic or stereotypic behaviour in young rats. Our compound improved the performance of aged but not young rats in a reward-motivated task. The well-described impairment of the dopaminergic system in aging may underlie the age-specific effect. We propose S-CE-123 as a possible candidate for developing a tentative therapeutic strategy for age-related cognitive decline and cognitive dysfunction in psychiatric disorders.
Keywords: DAT; aging; dopamine; learning and memory; reward.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

