Oral Immunization with Escherichia coli Nissle 1917 Expressing SARS-CoV-2 Spike Protein Induces Mucosal and Systemic Antibody Responses in Mice
- PMID: 36979504
- PMCID: PMC10046078
- DOI: 10.3390/biom13030569
Oral Immunization with Escherichia coli Nissle 1917 Expressing SARS-CoV-2 Spike Protein Induces Mucosal and Systemic Antibody Responses in Mice
Abstract
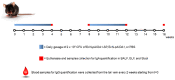
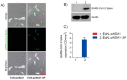
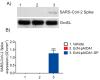
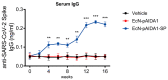
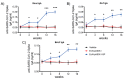
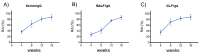
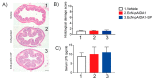
As of October 2022, the COVID-19 pandemic continues to pose a major public health conundrum, with increased rates of symptomatic infections in vaccinated individuals. An ideal vaccine candidate for the prevention of outbreaks should be rapidly scalable, easy to administer, and able to elicit a potent mucosal immunity. Towards this aim, we proposed an engineered Escherichia coli (E. coli) Nissle 1917 (EcN) strain with SARS-CoV-2 spike protein (SP)-coding plasmid, which was able to expose SP on its cellular surface by a hybridization with the adhesin involved in diffuse adherence 1 (AIDA1). In this study, we presented the effectiveness of a 16-week intragastrically administered, engineered EcN in producing specific systemic and mucosal immunoglobulins against SARS-CoV-2 SP in mice. We observed a time-dependent increase in anti-SARS-CoV-2 SP IgG antibodies in the sera at week 4, with a titre that more than doubled by week 12 and a stable circulating titre by week 16 (+309% and +325% vs. control; both p < 0.001). A parallel rise in mucosal IgA antibody titre in stools, measured via intestinal and bronchoalveolar lavage fluids of the treated mice, reached a plateau by week 12 and until the end of the immunization protocol (+300, +47, and +150%, at week 16; all p < 0.001 vs. controls). If confirmed in animal models of infection, our data indicated that the engineered EcN may be a potential candidate as an oral vaccine against COVID-19. It is safe, inexpensive, and, most importantly, able to stimulate the production of both systemic and mucosal anti-SARS-CoV-2 spike-protein antibodies.
Keywords: COVID-19; IgA; engineered probiotics; oral vaccine.
Conflict of interest statement
The authors declare no competing interests. Giuseppe Esposito, Giovanni Esposito, Walter Sanseverino, and Giovanni Sarnelli are all affiliated with Nextbiomics s.r.l., Naples, Italy. Nextbiomics s.r.l is an academic off-shoot of the University “Federico II” of Naples and should not, therefore, be perceived as a commercial conflict of interest.
Figures

Similar articles
-
Engineered bacteria as an orally administered anti-viral treatment and immunization system.Gut Microbes. 2025 Dec;17(1):2500056. doi: 10.1080/19490976.2025.2500056. Epub 2025 May 8. Gut Microbes. 2025. PMID: 40340796 Free PMC article.
-
Intranasal administration of Escherichia coli Nissle expressing the spike protein of SARS-CoV-2 induces long-term immunization and prevents spike protein-mediated lung injury in mice.Biomed Pharmacother. 2024 May;174:116441. doi: 10.1016/j.biopha.2024.116441. Epub 2024 Mar 21. Biomed Pharmacother. 2024. PMID: 38518597
-
Peritoneal Administration of a Subunit Vaccine Encapsulated in a Nanodelivery System Not Only Augments Systemic Responses against SARS-CoV-2 but Also Stimulates Responses in the Respiratory Tract.Viruses. 2021 Nov 2;13(11):2202. doi: 10.3390/v13112202. Viruses. 2021. PMID: 34835008 Free PMC article.
-
Oral subunit SARS-CoV-2 vaccine induces systemic neutralizing IgG, IgA and cellular immune responses and can boost neutralizing antibody responses primed by an injected vaccine.Vaccine. 2022 Feb 16;40(8):1098-1107. doi: 10.1016/j.vaccine.2022.01.025. Epub 2022 Jan 19. Vaccine. 2022. PMID: 35078662 Free PMC article.
-
[The Challenges of Vaccine Development Against Betacoronaviruses: Antibody Dependent Enhancement and Sendai Virus as a Possible Vaccine Vector].Mol Biol (Mosk). 2020 Nov-Dec;54(6):922-938. doi: 10.31857/S0026898420060154. Mol Biol (Mosk). 2020. PMID: 33276356 Review. Russian.
Cited by
-
Engineered bacteria as an orally administered anti-viral treatment and immunization system.Gut Microbes. 2025 Dec;17(1):2500056. doi: 10.1080/19490976.2025.2500056. Epub 2025 May 8. Gut Microbes. 2025. PMID: 40340796 Free PMC article.
-
Gut Microbiota-Based Immunotherapy: Engineered Escherichia coli Nissle 1917 for Oral Delivery of Glypican-1 in Pancreatic Cancer.Medicina (Kaunas). 2025 Mar 30;61(4):633. doi: 10.3390/medicina61040633. Medicina (Kaunas). 2025. PMID: 40282924 Free PMC article.
-
Escherichia coli Nissle 1917 efficiently expresses the RBD domain of SARS-CoV-2 spike protein without codon optimization.Sci Rep. 2025 May 5;15(1):15670. doi: 10.1038/s41598-025-99902-z. Sci Rep. 2025. PMID: 40325187 Free PMC article.
-
Modulation of oral vaccine efficacy by the gut microbiota.NPJ Vaccines. 2025 Aug 1;10(1):179. doi: 10.1038/s41541-025-01240-8. NPJ Vaccines. 2025. PMID: 40750603 Free PMC article. Review.
-
Engineering Adhesion of the Probiotic Strain Escherichia coli Nissle to the Fungal Pathogen Candida albicans.ACS Synth Biol. 2024 Dec 20;13(12):4027-4039. doi: 10.1021/acssynbio.4c00466. Epub 2024 Sep 12. ACS Synth Biol. 2024. PMID: 39265099 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

