Magnetite-Based Biosensors and Molecular Logic Gates: From Magnetite Synthesis to Application
- PMID: 36979516
- PMCID: PMC10046048
- DOI: 10.3390/bios13030304
Magnetite-Based Biosensors and Molecular Logic Gates: From Magnetite Synthesis to Application
Abstract
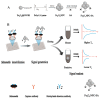
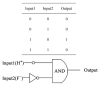
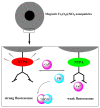
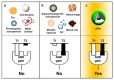
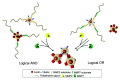
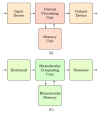
In the last few decades, point-of-care (POC) sensors have become increasingly important in the detection of various targets for the early diagnostics and treatment of diseases. Diverse nanomaterials are used as building blocks for the development of smart biosensors and magnetite nanoparticles (MNPs) are among them. The intrinsic properties of MNPs, such as their large surface area, chemical stability, ease of functionalization, high saturation magnetization, and more, mean they have great potential for use in biosensors. Moreover, the unique characteristics of MNPs, such as their response to external magnetic fields, allow them to be easily manipulated (concentrated and redispersed) in fluidic media. As they are functionalized with biomolecules, MNPs bear high sensitivity and selectivity towards the detection of target biomolecules, which means they are advantageous in biosensor development and lead to a more sensitive, rapid, and accurate identification and quantification of target analytes. Due to the abovementioned properties of functionalized MNPs and their unique magnetic characteristics, they could be employed in the creation of new POC devices, molecular logic gates, and new biomolecular-based biocomputing interfaces, which would build on new ideas and principles. The current review outlines the synthesis, surface coverage, and functionalization of MNPs, as well as recent advancements in magnetite-based biosensors for POC diagnostics and some perspectives in molecular logic, and it also contains some of our own results regarding the topic, which include synthetic MNPs, their application for sample preparation, and the design of fluorescent-based molecular logic gates.
Keywords: biosensors; magnetite nanoparticles (MNPs); molecular logic; point-of-care testing; surface functionalization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Losito D.W., de Araujo D.R., Bezzon V.D.N., Filho P.L.O., Fonseca F.L.A., dos Santos Chagas C., Barbosa E., Oliveira C.L.P., de Abreu Fantini M.C., Ferreira F.F., et al. Mesoporous Silica-Fe3O4 Nanoparticle Composites as Potential Drug Carriers. ACS Appl. Nano. Mater. 2021;4:13363–13378. doi: 10.1021/acsanm.1c02861. - DOI
-
- Bia Q., Song X., Hu A., Luo T., Jin R., Ai H., Nie Y. Magnetofection: Magic magnetic nanoparticles for efficient gene delivery. Chin. Chem. Lett. 2020;31:3041–3046. doi: 10.1016/j.cclet.2020.07.030. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

