Co-Culture of Glomerular Endothelial Cells and Podocytes in a Custom-Designed Glomerulus-on-a-Chip Model Improves the Filtration Barrier Integrity and Affects the Glomerular Cell Phenotype
- PMID: 36979551
- PMCID: PMC10046631
- DOI: 10.3390/bios13030339
Co-Culture of Glomerular Endothelial Cells and Podocytes in a Custom-Designed Glomerulus-on-a-Chip Model Improves the Filtration Barrier Integrity and Affects the Glomerular Cell Phenotype
Abstract
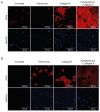
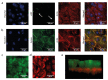
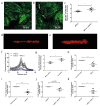
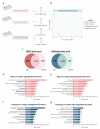
Crosstalk between glomerular endothelial cells and glomerular epithelial cells (podocytes) is increasingly becoming apparent as a crucial mechanism to maintain the integrity of the glomerular filtration barrier. However, in vitro studies directly investigating the effect of this crosstalk on the glomerular filtration barrier are scarce because of the lack of suitable experimental models. Therefore, we developed a custom-made glomerulus-on-a-chip model recapitulating the glomerular filtration barrier, in which we investigated the effects of co-culture of glomerular endothelial cells and podocytes on filtration barrier function and the phenotype of these respective cell types. The custom-made glomerulus-on-a-chip model was designed using soft lithography. The chip consisted of two parallel microfluidic channels separated by a semi-permeable polycarbonate membrane. The glycocalyx was visualized by wheat germ agglutinin staining and the barrier integrity of the glomerulus-on-a-chip model was determined by measuring the transport rate of fluorescently labelled dextran from the top to the bottom channel. The effect of crosstalk on the transcriptome of glomerular endothelial cells and podocytes was investigated via RNA-sequencing. Glomerular endothelial cells and podocytes were successfully cultured on opposite sides of the membrane in our glomerulus-on-a-chip model using a polydopamine and collagen A double coating. Barrier integrity of the chip model was significantly improved when glomerular endothelial cells were co-cultured with podocytes compared to monocultures of either glomerular endothelial cells or podocytes. Co-culture enlarged the surface area of podocyte foot processes and increased the thickness of the glycocalyx. RNA-sequencing analysis revealed the regulation of cellular pathways involved in cellular differentiation and cellular adhesion as a result of the interaction between glomerular endothelial cells and podocytes. We present a novel custom-made glomerulus-on-a-chip co-culture model and demonstrated for the first time using a glomerulus-on-a-chip model that co-culture affects the morphology and transcriptional phenotype of glomerular endothelial cells and podocytes. Moreover, we showed that co-culture improves barrier function as a relevant functional readout for clinical translation. This model can be used in future studies to investigate specific glomerular paracrine pathways and unravel the role of glomerular crosstalk in glomerular (patho) physiology.
Keywords: biological barrier; co-culture; crosstalk; glomerular endothelial cells; glomerular filtration barrier; glomerulus; glomerulus-on-a-chip; organ-on-a-chip; podocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Characterizing Glomerular Barrier Dysfunction with Patient-Derived Serum in Glomerulus-on-a-Chip Models: Unveiling New Insights into Glomerulonephritis.Int J Mol Sci. 2024 May 8;25(10):5121. doi: 10.3390/ijms25105121. Int J Mol Sci. 2024. PMID: 38791159 Free PMC article.
-
Glomerular filtration barrier modeling on a chip with tunable basement membrane deposition and 3D cultured podocytes.Lab Chip. 2023 Jul 25;23(15):3501-3517. doi: 10.1039/d3lc00147d. Lab Chip. 2023. PMID: 37432664
-
Loss of the Endothelial Glycocalyx Component EMCN Leads to Glomerular Impairment.Circ Res. 2025 Jan 3;136(1):59-74. doi: 10.1161/CIRCRESAHA.124.325218. Epub 2024 Nov 25. Circ Res. 2025. PMID: 39584795 Free PMC article.
-
Glomerulus-on-a-Chip: Current Insights and Future Potential Towards Recapitulating Selectively Permeable Filtration Systems.Int J Nephrol Renovasc Dis. 2022 Mar 10;15:85-101. doi: 10.2147/IJNRD.S344725. eCollection 2022. Int J Nephrol Renovasc Dis. 2022. PMID: 35299832 Free PMC article. Review.
-
Modeling the Glomerular Filtration Barrier and Intercellular Crosstalk.Front Physiol. 2021 Jun 2;12:689083. doi: 10.3389/fphys.2021.689083. eCollection 2021. Front Physiol. 2021. PMID: 34149462 Free PMC article. Review.
Cited by
-
Recent Methods for Modifying Mechanical Properties of Tissue-Engineered Scaffolds for Clinical Applications.Biomimetics (Basel). 2023 May 16;8(2):205. doi: 10.3390/biomimetics8020205. Biomimetics (Basel). 2023. PMID: 37218791 Free PMC article. Review.
-
Effects of steroid-resistant nephrotic syndrome serum on AA pathway in podocytes cultured in 3D in vitro glomerular model.Sci Rep. 2025 Apr 14;15(1):12802. doi: 10.1038/s41598-025-95216-2. Sci Rep. 2025. PMID: 40229314 Free PMC article.
-
Transplantation of decellularized porcine kidney grafts repopulated with primary human cells demonstrates filtration function in pigs.Commun Med (Lond). 2024 Dec 5;4(1):259. doi: 10.1038/s43856-024-00676-8. Commun Med (Lond). 2024. PMID: 39639166 Free PMC article.
-
Crosstalk among podocytes, glomerular endothelial cells and mesangial cells in diabetic kidney disease: an updated review.Cell Commun Signal. 2024 Feb 19;22(1):136. doi: 10.1186/s12964-024-01502-3. Cell Commun Signal. 2024. PMID: 38374141 Free PMC article. Review.
-
Characterizing Glomerular Barrier Dysfunction with Patient-Derived Serum in Glomerulus-on-a-Chip Models: Unveiling New Insights into Glomerulonephritis.Int J Mol Sci. 2024 May 8;25(10):5121. doi: 10.3390/ijms25105121. Int J Mol Sci. 2024. PMID: 38791159 Free PMC article.
References
-
- Rops A.L., Loeven M.A., van Gemst J.J., Eversen I., Van Wijk X.M., Dijkman H.B., van Kuppevelt T.H., Berden J.H., Rabelink T.J., Esko J.D., et al. Modulation of heparan sulfate in the glomerular endothelial glycocalyx decreases leukocyte influx during experimental glomerulonephritis. Kidney Int. 2014;86:932–942. doi: 10.1038/ki.2014.115. - DOI - PubMed
-
- Garsen M., Lenoir O., Rops A.L., Dijkman H.B., Willemsen B., van Kuppevelt T.H., Rabelink T.J., Berden J.H., Tharaux P.L., van der Vlag J. Endothelin-1 Induces Proteinuria by Heparanase-Mediated Disruption of the Glomerular Glycocalyx. J. Am. Soc. Nephrol. 2016;27:3545–3551. doi: 10.1681/ASN.2015091070. - DOI - PMC - PubMed
-
- Van den Berg B.M., Wang G., Boels M.G.S., Avramut M.C., Jansen E., Sol W., Lebrin F., van Zonneveld A.J., de Koning E.J.P., Vink H., et al. Glomerular Function and Structural Integrity Depend on Hyaluronan Synthesis by Glomerular Endothelium. J. Am. Soc. Nephrol. 2019;30:1886–1897. doi: 10.1681/ASN.2019020192. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

