A New, Extremely Sensitive, Turn-Off Optical Sensor Utilizing Schiff Base for Fast Detection of Cu(II)
- PMID: 36979571
- PMCID: PMC10046006
- DOI: 10.3390/bios13030359
A New, Extremely Sensitive, Turn-Off Optical Sensor Utilizing Schiff Base for Fast Detection of Cu(II)
Abstract
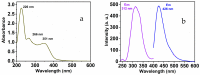
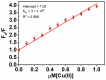
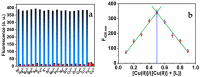
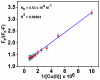
Throughout this research, a unique optical sensor for detecting one of the most dangerous heavy metal ions, Cu(II), was designed and developed. The (4-mercaptophenyl) iminomethylphenyl naphthalenyl carbamate (MNC) sensor probe was effectively prepared. The Schiff base of the sensor shows a "turn-off" state with excellent sensitivity to Cu(II) ions. This innovative fluorescent chemosensor possesses distinctive optical features with a substantial Stocks shift (about 114 nm). In addition, MNC has remarkable selectivity for Cu(II) relative to other cations. Density functional theory (DFT) and the time-dependent DFT (TDDFT) theoretical calculations were performed to examine Cu(II) chelation structures and associated electronic properties in solution, and the results indicate that the luminescence quenching in this complex is due to ICT. Chelation-quenched fluorescence is responsible for the internal charge transfer (ICT)-based selectivity of the MNC sensing molecule for Cu(II) ions. In a 1:9 (v/v) DMSO-HEPES buffer (20 mM, pH = 7.4) solution, Fluorescence and UV-Vis absorption of the MNC probe and Cu(II) ions were investigated. By utilizing a solution containing several metal ions, the interference of other metal ions was studied. This MNC molecule has outstanding selectivity and sensitivity, as well as a low LOD (1.45 nM). Consequently, these distinctive properties enable it to find the copper metal ions across an actual narrow dynamic range (0-1.2 M Cu(II)). The reversibility of the sensor was obtained by employing an EDTA as a powerful chelating agent.
Keywords: chemical analysis; copper; fluorescence; optical properties; sensor; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Pietrini F., Carnevale M., Beni C., Zacchini M., Gallucci F., Santangelo E. Effect of different copper levels on growth and morpho-physiological parameters in giant reed (Arundo donax L.) in semi-hydroponic mesocosm experiment. Water. 2019;11:1837. doi: 10.3390/w11091837. - DOI
-
- Oliveri V. Biomedical applications of copper ionophores. Coord. Chem. Rev. 2020;422:213474. doi: 10.1016/j.ccr.2020.213474. - DOI
-
- Ye X., Li Y., Luo P., He B., Cao X., Lu T. Iron sites on defective BiOBr nanosheets: Tailoring the molecular oxygen activation for enhanced photocatalytic organic synthesis. Nano Res. 2022;15:1509–1516. doi: 10.1007/s12274-021-3695-2. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

