Current Development of Chemical Penetration Enhancers for Transdermal Insulin Delivery
- PMID: 36979643
- PMCID: PMC10044980
- DOI: 10.3390/biomedicines11030664
Current Development of Chemical Penetration Enhancers for Transdermal Insulin Delivery
Abstract
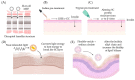
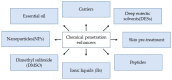
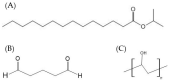
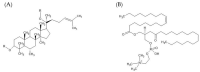
The use of the transdermal delivery system has recently gained ample recognition due to the ability to deliver drug molecules across the skin membrane, serving as an alternative to conventional oral or injectable routes. Subcutaneous insulin injection is the mainstay treatment for diabetes mellitus which often leads to non-compliance among patients, especially in younger patients. Apart from its invasiveness, the long-term consequences of insulin injection cause the development of physical trauma, which includes lipohypertrophy at the site of administration, scarring, infection, and sometimes nerve damage. Hence, there is a quest for a better alternative to drug delivery that is non-invasive and easily adaptable. One of the potential solutions is the transdermal delivery method. However, the stratum corneum (the top layer of skin) is the greatest barrier in transporting large molecules like insulin. Therefore, various chemical enhancers have been proposed to promote stratum corneum permeability, or they are designed to increase the permeability of the full epidermis, such as the use of ionic liquid, peptides, chemical pre-treatment as well as packaging insulin with carriers or nanoparticles. In this review, the recent progress in the development of chemical enhancers for transdermal insulin delivery is discussed along with the possible mechanistic of action and the potential outlook on the proposed permeation approaches in comparison to other therapeutical drugs.
Keywords: chemical enhancers; deep eutectic solvents; diabetes; emulsions; insulin; ionic liquid; nanoparticles; peptides; transdermal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Williams R., Colagiuri S., Chan J., Gregg E., Ke C., Lim L.-L., Yang X. IDF Atlas. 9th ed. 2019. [(accessed on 26 January 2023)]. Available online: https://diabetesatlas.org/atlas/ninth-edition/
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

