Association between Biomarkers (VEGF-R2, VEGF-R3, VCAM-1) and Treatment Duration in Patients with Neuroendocrine Tumors Receiving Therapy with First-Generation Somatostatin Analogues
- PMID: 36979820
- PMCID: PMC10044914
- DOI: 10.3390/biomedicines11030842
Association between Biomarkers (VEGF-R2, VEGF-R3, VCAM-1) and Treatment Duration in Patients with Neuroendocrine Tumors Receiving Therapy with First-Generation Somatostatin Analogues
Abstract
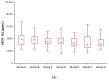
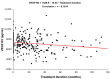
Angiogenic factors (AF) promote vascular formation and may thus support neuroendocrine tumour (NET) development. This study aimed to assess AF serum level changes in NET patients treated with prolonged-acting somatostatin analogues (SSAs). The study enrolled 49 healthy volunteers (Group A) and 56 NET patients: treatment naïve (Group B) and after-SSA treatment in various periods (months): under 12 (Group C), 13-24 (Group D), 25-36 (Group E), 37-60 (Group F), and over 60 months (Group G). The serum vascular endothelial growth factor receptors 2, 3 (VEGF-R2, VEGF-R3), and vascular cell adhesion molecule-1 (VCAM-1) concentrations were tested using the ELISA. We noted significant differences in the concentrations of VEGF-R2, VEGF-R3, and VCAM-1 depending on the SSA treatment duration (p < 0.001). In the studied AFs, the highest decreasing levels of VEGF-R2 were observed after two years of therapy. However, monitoring VEGF-R2, VEGF-R3, and VCAM-1 during SSA treatment did not allow for the identification of good responders for this kind of therapy. Therefore, these biomarker measurements were not helpful in assessing SSA treatment effectiveness in NET patients.
Keywords: VCAM-1; VEGF-R2; VEGF-R3; neuroendocrine tumors; somatostatin analogues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kos-Kudła B., Foltyn W., Malczewska A., Bednarczuk T., Bolanowski M., Borowska M., Chmielik E., Ćwikła J.B., Gisterek I., Handkiewicz-Junak D., et al. Update of the diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours) Endokrynol. Pol. 2022;73:387–454. doi: 10.5603/EP.a2022.0049. - DOI - PubMed
-
- Kos-Kudła B., Blicharz-Dorniak J., Strzelczyk J., Bałdys-Waligórska A., Bednarczuk T., Bolanowski M., Boratyn-Nowicka A., Borowska M., Cichocki A., Ćwikła J.B., et al. Diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours) Endokrynol. Pol. 2017;68:79–110. doi: 10.5603/EP.2017.0015. - DOI - PubMed
-
- Bednarczuk T., Zemczak A., Bolanowski M., Borowska M., Chmielik E., Ćwikła J.B., Foltyn W., Gisterek I., Handkiewicz-Junak D., Hubalewska-Dydejczyk A., et al. Neuroendocrine neoplasms of the small intestine and the appendix–update of the diagnostic and therapeutic guidelines (recommended by the Polish Network of Neuroendocrine Tumours) Endokrynol. Pol. 2022;73:549–583. doi: 10.5603/EP.a2022.0052. - DOI - PubMed
-
- Pavel M., Valle J.W., Eriksson B., Rinke A., Caplin M., Chen J., Costa F., Falkerby J., Fazio N., Gorbounova V., et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms: Systemic Therapy–Biotherapy and Novel Targeted Agents. Neuroendocrinology. 2017;105:266–280. doi: 10.1159/000471880. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

