Identification and Elimination of Antifungal Tolerance in Candida auris
- PMID: 36979876
- PMCID: PMC10045952
- DOI: 10.3390/biomedicines11030898
Identification and Elimination of Antifungal Tolerance in Candida auris
Abstract
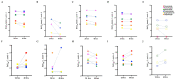
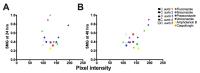
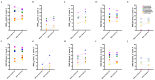
Antimicrobial resistance is a global health crisis to which pathogenic fungi make a substantial contribution. The human fungal pathogen C. auris is of particular concern due to its rapid spread across the world and its evolution of multidrug resistance. Fluconazole failure in C. auris has been recently attributed to antifungal "tolerance". Tolerance is a phenomenon whereby a slow-growing subpopulation of tolerant cells, which are genetically identical to susceptible cells, emerges during drug treatment. We use microbroth dilution and disk diffusion assays, together with image analysis, to investigate antifungal tolerance in C. auris to all three classes of antifungal drugs used to treat invasive candidiasis. We find that (1) C. auris is tolerant to several common fungistatic and fungicidal drugs, which in some cases can be detected after 24 h, as well as after 48 h, of antifungal drug exposure; (2) the tolerant phenotype reverts to the susceptible phenotype in C. auris; and (3) combining azole, polyene, and echinocandin antifungal drugs with the adjuvant chloroquine in some cases reduces or eliminates tolerance and resistance in patient-derived C. auris isolates. These results suggest that tolerance contributes to treatment failure in C. auris infections for a broad range of antifungal drugs, and that antifungal adjuvants may improve treatment outcomes for patients infected with antifungal-tolerant or antifungal-resistant fungal pathogens.
Keywords: Candida auris; adjuvant; antifungal tolerance/resistance; broth microdilution assay; disk diffusion assay; diskImageR; human fungal pathogen.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Andes D.R., Safdar N., Baddley J.W., Alexander B., Brumble L., Freifeld A., Hadley S., Herwaldt L., Kauffman C., Lyon G.M., et al. The Epidemiology and Outcomes of Invasive Candida Infections among Organ Transplant Recipients in the United States: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Transpl. Infect. Dis. 2016;18:921–931. doi: 10.1111/tid.12613. - DOI - PubMed
-
- O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations: The Review on Antimicrobial Resistanc. 2016. [(accessed on 10 November 2022)]. Available online: https://Amr-Review.Org.
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

