Effect of Ouabain on Glutamate Transport in the Hippocampus of Rats with LPS-Induced Neuroinflammation
- PMID: 36979899
- PMCID: PMC10045517
- DOI: 10.3390/biomedicines11030920
Effect of Ouabain on Glutamate Transport in the Hippocampus of Rats with LPS-Induced Neuroinflammation
Abstract
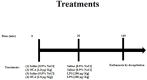
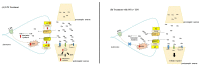
A lipopolysaccharide (LPS)-induced neuroinflammation rat model was used to study the effects of ouabain (OUA) at low concentrations, which can interact with the Na,K-ATPase, causing the modulation of intracellular signalling pathways in the Central Nervous System. Our study aimed to analyse the effects of OUA on glutamate transport in the hippocampus of rats with LPS-induced neuroinflammation. Adult male Wistar rats were divided into four groups: OUA (1.8 µg/kg), saline (CTR), LPS (200 µg/kg), and OUA + LPS (OUA 20 min before LPS). The animals were sacrificed after 2 h, and the hippocampus was collected for analysis. After treatment, we determined the activities of Na,K-ATPase and glutamine synthetase (GS). In addition, expression of the α1, α2, and α3 isoforms of Na,K-ATPase and the glutamate transporters, EAAT1 and EAAT2, were also analysed. Treatment with OUA caused a specific increase in the α2 isoform expression (~20%), whereas LPS decreased its expression (~22%), and treatment with OUA before LPS prevented the effects of LPS. Moreover, LPS caused a decrease of approximately 50% in GS activity compared with that in the CTR group; however, OUA pre-treatment attenuated this effect of LPS. Notably, it was found that treatment with OUA caused an increase in the expression of EAAT1 (~30%) and EAAT2 (~25%), whereas LPS caused a decrease in the expression of EAAT1 (~23%) and EAAT2 (~25%) compared with that in the CTR group. When treated with OUA, the effects of LPS were abrogated. In conclusion, the OUA pre-treatment abolished the effect caused by LPS, suggesting that this finding may be related to the restoration of the interaction between FXYD2 and the studied membrane proteins.
Keywords: EAATs; FXYD2; K-ATPase; LPS; Na; glutamate; hippocampus; ouabain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
- PPM-00307-18/Fundação de Amparo à Pesquisa do Estado de Minas Gerais
- APQ-00855-19/Fundação de Amparo à Pesquisa do Estado de Minas Gerais
- 305173/2018-9/National Council for Scientific and Technological Development
- 401914/2016-0/National Council for Scientific and Technological Development
- 2014/01425-3/São Paulo Research Foundation
- 2016/07427-8/São Paulo Research Foundation
- code 01/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- NAPNA/USP Neuroscience Research Support Centres
- 305441/2021-3/National Council for Scientific and Technological Development
- 403646/2021-9/National Council for Scientific and Technological Development
- APQ 01915-22/Fundação de Amparo à Pesquisa do Estado de Minas Gerais
LinkOut - more resources
Full Text Sources

