Structure-Guided Prediction of the Functional Impact of DCLK1 Mutations on Tumorigenesis
- PMID: 36979969
- PMCID: PMC10046695
- DOI: 10.3390/biomedicines11030990
Structure-Guided Prediction of the Functional Impact of DCLK1 Mutations on Tumorigenesis
Abstract
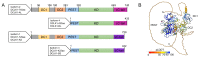
Doublecortin-like kinase 1 (DCLK1) is a functional serine/threonine (S/T)-kinase and a member of the doublecortin family of proteins which are characterized by their ability to bind to microtubules (MTs). DCLK1 is a proposed cancer driver gene, and its upregulation is associated with poor overall survival in several solid cancer types. However, how DCLK1 associates with MTs and how its kinase function contributes to pro-tumorigenic processes is poorly understood. This review builds on structural models to propose not only the specific functions of the domains but also attempts to predict the impact of individual somatic missense mutations on DCLK1 functions. Somatic missense mutations in DCLK1 are most frequently located within the N-terminal MT binding region and likely impact on the ability of DCLK1 to bind to αβ-tubulin and to polymerize and stabilize MTs. Moreover, the MT binding affinity of DCLK1 is negatively regulated by its auto-phosphorylation, and therefore mutations that affect kinase activity are predicted to indirectly alter MT dynamics. The emerging picture portrays DCLK1 as an MT-associated protein whose interactions with tubulin heterodimers and MTs are tightly controlled processes which, when disrupted, may confer pro-tumorigenic properties.
Keywords: DCLK1; DCX; PEST domain; cancer; cryo-EM; crystal structure; doublecortin domain; kinase; microtubules; missense mutations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

