Prognostic Role of Soluble and Extracellular Vesicle-Associated PD-L1, B7-H3 and B7-H4 in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors
- PMID: 36980174
- PMCID: PMC10047289
- DOI: 10.3390/cells12060832
Prognostic Role of Soluble and Extracellular Vesicle-Associated PD-L1, B7-H3 and B7-H4 in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors
Abstract
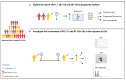
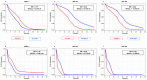
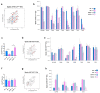
The treatment of non-small cell lung cancer (NSCLC) has changed dramatically with the advent of immune checkpoint inhibitors (ICIs). Despite encouraging results, their efficacy remains limited to a subgroup of patients. Circulating immune checkpoints in soluble (s) form and associated with extracellular vesicles (EVs) represent promising markers, especially in ICI-based therapeutic settings. We evaluated the prognostic role of PD-L1 and of two B7 family members (B7-H3, B7-H4), both soluble and EV-associated, in a cohort of advanced NSCLC patients treated with first- (n = 56) or second-line (n = 126) ICIs. In treatment-naïve patients, high baseline concentrations of sPD-L1 (>24.2 pg/mL) were linked to worse survival, whereas high levels of sB7-H3 (>0.5 ng/mL) and sB7-H4 (>63.9 pg/mL) were associated with better outcomes. EV characterization confirmed the presence of EVs positive for PD-L1 and B7-H3, while only a small portion of EVs expressed B7-H4. The comparison between biomarker levels at the baseline and in the first radiological assessment under ICI-based treatment showed a significant decrease in EV-PD-L1 and an increase in EV-B7H3 in patients in the disease response to ICIs. Our study shows that sPD-L1, sB7-H3 and sB7-H4 levels are emerging prognostic markers in patients with advanced NSCLC treated with ICIs and suggests potential EV involvement in the disease response to ICIs.
Keywords: B7-H3; B7-H4; NSCLC; PD-L1; extracellular vesicle; immune checkpoint inhibitors; platelets; prognosis; soluble protein.
Conflict of interest statement
C.G. (Carlo Genova) declares honoraria from Amgen, AstraZeneca, Bristol-Myers-Squibb, Eli-Lilly, Merck Sharp and Dohme, Novartis, Roche, Sanofi, Takeda and ThermoFisher. Research grants from: Bristol-Myers Squibb and Italian Ministry of Health. G.R. declares honoraria from AstraZeneca, Bristol-Myers Squibb, Roche, MSD and Janssen. C.D. declares honoraria from Bristol-Myers Squibb, Roche and Astra Zeneca. M.T. declares honoraria for travel, accommodation, expenses from Roche, Bristol-Myers Squibb, AstraZeneca, Takeda and Eli Lilly. Honoraria as medical writers: Novartis, Amgen and MSD. G.B. declares honoraria from AstraZeneca, Roche, Pierre Fabre and GSK. E.R. declares a travel grant from: daichii sankyo and honoraria from BMS, SANOFI, ASTRA ZENECA, MSD and ROCHE. F.G. declares his advisory role in ad hoc advisory boards/consultations (last 3 years); honoraria from Eli Lilly, Roche, Boehringer Ingelheim, AstraZeneca, Pierre Fabre, BMS, MSD, Novartis, Merck, Otsuka, Novartis and Takeda; Honoraria for seminars/talks to the industry (last 3 years): Eli Lilly, Roche, Boehringer Ingelheim, AstraZeneca, Pierre Fabre, AMGEN, Celgene, BMS and MSD; Research Funding (last 3 years): AstraZeneca, BMS and MSD. The other authors declare no conflict of interest.
Figures


References
-
- Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. - DOI - PMC - PubMed
-
- Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., Gadgeel S.M., Hida T., Kowalski D.M., Dols M.C., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. - DOI - PMC - PubMed
-
- Herbst R.S., Garon E.B., Kim D.W., Cho B.C., Gervais R., Perez-Gracia J.L., Han J.Y., Majem M., Forster M.D., Monnet I., et al. Five Year Survival Update From KEYNOTE-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J. Thorac. Oncol. 2021;16:1718–1732. doi: 10.1016/j.jtho.2021.05.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

