Mitochondrial Haemoglobin Is Upregulated with Hypoxia in Skeletal Muscle and Has a Conserved Interaction with ATP Synthase and Inhibitory Factor 1
- PMID: 36980252
- PMCID: PMC10047868
- DOI: 10.3390/cells12060912
Mitochondrial Haemoglobin Is Upregulated with Hypoxia in Skeletal Muscle and Has a Conserved Interaction with ATP Synthase and Inhibitory Factor 1
Abstract
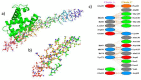
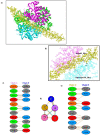
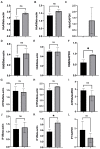
The globin protein superfamily has diverse functions. Haemoglobin has been found in non-erythroid locations, including within the mitochondria. Using co-immunoprecipitation and in silico methods, we investigated the interaction of mitochondrial haemoglobin with ATP synthase and its associated proteins, including inhibitory factor 1 (IF1). We measured the expression of mitochondrial haemoglobin in response to hypoxia. In vitro and in silico evidence of interactions between mitochondrial haemoglobin and ATP synthase were found, and we report upregulated mitochondrial haemoglobin expression in response to hypoxia within skeletal muscle tissue. Our observations indicate that mitochondrial pH and ATP synthase activity are implicated in the mitochondrial haemoglobin response to hypoxia.
Keywords: ATP synthase; ATP synthase inhibitory factor 1; haemoglobin; hypoxia; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Thomas C., Lumb A.B. Physiology of haemoglobin. Contin. Educ. Anaesth. Crit. Care Pain. 2012;12:251–256. doi: 10.1093/bjaceaccp/mks025. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

