Impact of Omicron Variant Infection on Assessment of Spike-Specific Immune Responses Using the EUROIMMUN Quan-T-Cell SARS-CoV-2 Assay and Roche Elecsys Anti-SARS-CoV-2-S
- PMID: 36980332
- PMCID: PMC10047097
- DOI: 10.3390/diagnostics13061024
Impact of Omicron Variant Infection on Assessment of Spike-Specific Immune Responses Using the EUROIMMUN Quan-T-Cell SARS-CoV-2 Assay and Roche Elecsys Anti-SARS-CoV-2-S
Abstract
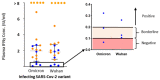
The currently prevailing variants of SARS-CoV-2 are subvariants of the Omicron variant. The aim of this study was to analyze the effect of mutations in the Spike protein of Omicron on the results Quan-T-Cell SARS-CoV-2 assays and Roche Elecsys anti-SARS-CoV-2 anti-S1. Omicron infected subjects ((n = 37), vaccinated (n = 20) and unvaccinated (n = 17)) were recruited approximately 3 weeks after a positive PCR test. The Quan-T-Cell SARS-CoV-2 assays (EUROIMMUN) using Wuhan and the Omicron adapted antigen assay and a serological test (Roche Elecsys anti-SARS-CoV-2 anti-S1) were performed. Using the original Wuhan SARS-CoV-2 IGRA TUBE, in 19 of 21 tested Omicron infected subjects, a positive IFNy response was detected, while 2 non-vaccinated but infected subjects did not respond. The Omicron adapted antigen tube resulted in comparable results. In contrast, the serological assay detected a factor 100-fold lower median Spike-specific RBD antibody concentration in non-vaccinated Omicron infected patients (n = 12) compared to patients from the pre Omicron era (n = 12) at matched time points, and eight individuals remained below the detection threshold for positivity. For vaccinated subjects, the Roche assay detected antibodies in all subjects and showed a 400 times higher median specific antibody concentration compared to non-vaccinated infected subjects in the pre-Omicron era. Our results suggest that Omicron antigen adapted IGRA stimulator tubes did not improve detection of SARS-CoV-2-specific T-cell responses in the Quant-T-Cell-SARS-CoV-2 assay. In non-vaccinated Omicron infected individuals, the Wuhan based Elecsys anti-SARS-CoV-2 anti-S1 serological assay results in many negative results at 3 weeks after diagnosis.
Keywords: SARS-CoV-2; breakthrough infections; omicron; spike-specific immune response.
Conflict of interest statement
D.Z. is employed by EUROIMMUN, a manufacturer of diagnostic reagents and co-owner of patents related to serological assays for the diagnosis of SARS-CoV-2 and the detection of immunity as a result of vaccination. EUROIMMUN and Roche provided kits and machines for analyses at discounted rates. The authors declare no conflict of interest.
Figures

References
-
- Wolter N., Jassat W., Walaza S., Welch R., Moultrie H., Groome M., Amoako D.G., Everatt J., Bhiman J.N., Scheepers C., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. - DOI - PMC - PubMed
-
- Puchinger K., Castelletti N., Rubio-Acero R., Geldmacher C., Eser T.M., Deák F., Paunovic I., Bakuli A., Saathoff E., von Meyer A., et al. The interplay of viral loads, clinical presentation, and serological responses in SARS-CoV-2–Results from a prospective cohort of outpatient COVID-19 cases. Virology. 2022;569:37–43. doi: 10.1016/j.virol.2022.02.002. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

