Performance of VIDAS® Diagnostic Tests for the Automated Detection of Dengue Virus NS1 Antigen and of Anti-Dengue Virus IgM and IgG Antibodies: A Multicentre, International Study
- PMID: 36980445
- PMCID: PMC10047366
- DOI: 10.3390/diagnostics13061137
Performance of VIDAS® Diagnostic Tests for the Automated Detection of Dengue Virus NS1 Antigen and of Anti-Dengue Virus IgM and IgG Antibodies: A Multicentre, International Study
Abstract
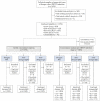
Dengue is a serious mosquito-transmitted disease caused by the dengue virus (DENV). Rapid and reliable diagnosis of DENV infection is urgently needed in dengue-endemic regions. We describe here the performance evaluation of the CE-marked VIDAS® dengue immunoassays developed for the automated detection of DENV NS1 antigen and anti-DENV IgM and IgG antibodies. A multicenter concordance study was conducted in 1296 patients from dengue-endemic regions in Asia, Latin America, and Africa. VIDAS® dengue results were compared to those of competitor enzyme-linked immunosorbent assays (ELISA). The VIDAS® dengue assays showed high precision (CV ≤ 10.7%) and limited cross-reactivity (≤15.4%) with other infections. VIDAS® DENGUE NS1 Ag showed high positive and negative percent agreement (92.8% PPA and 91.7% NPA) in acute patients within 0-5 days of symptom onset. VIDAS® Anti-DENGUE IgM and IgG showed a moderate-to-high concordance with ELISA (74.8% to 90.6%) in post-acute and recovery patients. PPA was further improved in combined VIDAS® NS1/IgM (96.4% in 0-5 days acute patients) and IgM/IgG (91.9% in post-acute patients) tests. Altogether, the VIDAS® dengue NS1, IgM, and IgG assays performed well, either alone or in combination, and should be suitable for the accurate diagnosis of DENV infection in dengue-endemic regions.
Keywords: DENV; ELISA; IgM and IgG antibodies; NS1 antigen; VIDAS®; dengue diagnosis.
Conflict of interest statement
L.B., L.D., F.S. and M.T. are employees of bioMérieux. M.L.N. and S.D. received compensation fees from bioMérieux for this study, in the framework of the agreement signed with their employer entity. This study was funded by bioMérieux. The funder was involved in the design and execution of the study, in the data interpretation, and in the writing of the manuscript.
Figures
References
-
- WHO, editor. Dengue: Guidelines for Diagnosis, Treatment, Prevention, and Control. World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases (TDR); Geneva, Switzerland: 2009. New edition.
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

