Phase I Trial of [99mTc]Tc-maSSS-PEG2-RM26, a Bombesin Analogue Antagonistic to Gastrin-Releasing Peptide Receptors (GRPRs), for SPECT Imaging of GRPR Expression in Malignant Tumors
- PMID: 36980517
- PMCID: PMC10046460
- DOI: 10.3390/cancers15061631
Phase I Trial of [99mTc]Tc-maSSS-PEG2-RM26, a Bombesin Analogue Antagonistic to Gastrin-Releasing Peptide Receptors (GRPRs), for SPECT Imaging of GRPR Expression in Malignant Tumors
Abstract
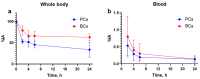
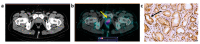
The gastrin-releasing peptide receptor (GRPR) is overexpressed in prostate cancer (PCa) and in hormone-driven breast cancer (BCa). The aim of this phase I clinical trial was to evaluate safety, biodistribution, and dosimetry after the administration of the recently developed GRPR-targeting antagonistic bombesin analogue [99mTc]Tc-maSSS-PEG2-RM26 in PCa and BCa patients. Planar and whole-body SPECT/CT imaging was performed in six PCa patients and seven BCa patients 2, 4, 6, and 24 h post the intravenous administration of 40 µg of [99mTc]Tc-maSSS-PEG2-RM26 (600-700 MBq). No adverse events or pathological changes were observed. The rapid blood clearance of [99mTc]Tc-maSSS-PEG2-RM26 was observed with predominantly hepatobiliary excretion. The effective doses were 0.0053 ± 0.0007 for male patients and 0.008 ± 0.003 mSv/MBq for female patients. The accumulation of [99mTc]Tc-maSSS-PEG2-RM26 in tumors was observed in four out of six PCa and in seven out of seven BCa patients. In four BCa patients, a high uptake of the agent into the axillary lymph nodes was detected. Immunohistochemistry revealed positive GRPR expression in 60% of primary PCa, 71.4% of BCa tumors, and 50% of examined BCa lymph nodes. In conclusion, a single administration of [99mTc]Tc-maSSS-PEG2-RM26 was safe and well tolerated. [99mTc]Tc-maSSS-PEG2-RM26 SPECT may be useful for tumor detection in PCa and BCa patients, pending further studies.
Keywords: 99mTc; GRPR; antagonist; phase I trial.
Conflict of interest statement
V.C., A.R., R.Z., A.M., O.B., N.L., E.U., A.A., S.S.R., J.S., V.T. and A.O. are co-applicants in patent 2776234 C1 (14.07.2022, application № 2021124249 12.08.2021)), Russian Federation. A.D., L.T. and S.V. declare no conflict of interest.
Figures


References
-
- Von Eyben F.E., Picchio M., von Eyben R., Rhee H., Bauman G. 68Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: A systematic review and meta-analysis. Eur. Urol. Focus. 2018;4:686–693. doi: 10.1016/j.euf.2016.11.002. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

