Biological Role and Clinical Implications of MYOD1L122R Mutation in Rhabdomyosarcoma
- PMID: 36980529
- PMCID: PMC10046495
- DOI: 10.3390/cancers15061644
Biological Role and Clinical Implications of MYOD1L122R Mutation in Rhabdomyosarcoma
Abstract
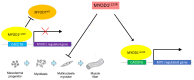
Major progress in recent decades has furthered our clinical and biological understanding of rhabdomyosarcoma (RMS) with improved stratification for treatment based on risk factors. Clinical risk factors alone were used to stratify patients for treatment in the European Pediatric Soft Tissue Sarcoma Study Group (EpSSG) RMS 2005 protocol. The current EpSSG overarching study for children and adults with frontline and relapsed rhabdomyosarcoma (FaR-RMS NCT04625907) includes FOXO1 fusion gene status in place of histology as a risk factor. Additional molecular features of significance have recently been recognized, including the MYOD1L122R gene mutation. Here, we review biological information showing that MYOD1L122R blocks cell differentiation and has a MYC-like activity that enhances tumorigenesis and is linked to an aggressive cellular phenotype. MYOD1L122R mutations can be found together with mutations in other genes, such as PIK3CA, as potentially cooperating events. Using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, ten publications in the clinical literature involving 72 cases were reviewed. MYOD1L122R mutation in RMS can occur in both adults and children and is frequent in sclerosing/spindle cell histology, although it is also significantly reported in a subset of embryonal RMS. MYOD1L122R mutated tumors most frequently arise in the head and neck and extremities and are associated with poor outcome, raising the issue of how to use MYOD1L122R in risk stratification and how to treat these patients most effectively.
Keywords: MYOD1; children; high-risk; sclerosing rhabdomyosarcoma; spindle cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bisogno G., De Salvo G.L., Bergeron C., Gallego Melcón S., Merks J.H., Kelsey A., Martelli H., Minard-Colin V., Orbach D., Glosli H., et al. Vinorelbine and continuous low-dose cyclophosphamide as maintenance chemotherapy in patients with high-risk rhabdomyosarcoma (RMS 2005): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20:1566–1575. doi: 10.1016/S1470-2045(19)30617-5. - DOI - PubMed
-
- Affinita M.C., Ferrari A., Chiaravalli S., Melchionda F., Quaglietta L., Casanova M., Zanetti I., Scarzello G., Di Pasquale L., Di Cataldo A., et al. Defining the impact of prognostic factors at the time of relapse for nonmetastatic rhabdomyosarcoma. Pediatr. Blood Cancer. 2020;67:e28674. doi: 10.1002/pbc.28674. - DOI - PubMed
-
- Chisholm J.C., Marandet J., Rey A., Scopinaro M., de Toledo J.S., Merks J.H.M., O’Meara A., Stevens M.C.G., Oberlin O. Prognostic factors after relapse in nonmetastatic rhabdomyosarcoma: A nomogram to better define patients who can be salvaged with further therapy. J. Clin. Oncol. 2011;29:1319–1325. doi: 10.1200/JCO.2010.32.1984. - DOI - PubMed
-
- Pappo A.S., Anderson J.R., Crist W.M., Wharam M.D., Breitfeld P.P., Hawkins D., Raney R.B., Womer R.B., Parham D.M., Qualman S.J., et al. Survival after Relapse in Children and Adolescents with Rhabdomyosarcoma: A Report from the Intergroup Rhabdomyosarcoma Study Group. J. Clin. Oncol. 1999;17:3487–3493. doi: 10.1200/JCO.1999.17.11.3487. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

