Cutaneous Squamous Cell Carcinoma in Immunocompromised Patients-A Comparison between Different Immunomodulating Conditions
- PMID: 36980651
- PMCID: PMC10046308
- DOI: 10.3390/cancers15061764
Cutaneous Squamous Cell Carcinoma in Immunocompromised Patients-A Comparison between Different Immunomodulating Conditions
Abstract
Background: Immunosuppression is strongly associated with an increased risk of developing cutaneous squamous cell carcinoma (cSCC). Studies on solid organ transplant recipients (SOTR) and chronic lymphocytic leukemia (CLL) patients have already demonstrated higher rates of aggressive cSCC tumors in these populations compared to immunocompetent controls. Studies on other immunosuppressed patient groups are scarce. This study was aimed at assessing the effects of different immunomodulating conditions on patients diagnosed with cSCC. We sought to compare the clinical features, treatments, and survival rates among the different study groups, as well as outcomes to those of immunocompetent controls with cSCC.
Methods: A retrospective analysis of 465 cSCC patients, both immunosuppressed (IS) and immunocompetent controls. Etiologies for immunosuppression included SOTR, CLL, chronic kidney disease (CKD), psoriasis, rheumatoid arthritis (RA) and systemic lupus erythematous (SLE).
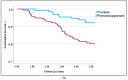
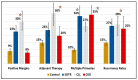
Results: Compared to the control group, IS patients demonstrated several significant differences. These include higher rates of positive resection margins, higher recurrence rates, and multiple SCC tumors. Patients in the IS group, who were also given immunomodulating agents, demonstrated even lower survival rates. Cox regression analysis demonstrated statistically significant decreased overall survival (OS) rates for IS patients compared to the controls (OR = 1.9, p = 0.031). SOTR patients tend to have multiple cSCC tumors (35%), with the highest number of primary tumors compared to controls (2.54 tumors per patient on average, p < 0.001), but also compared to all other IS groups. The average SCC lesion size in the SOTR group was the smallest, measuring at 13.5 mm, compared to the control group and all other IS groups. Decreased survival rates were seen on Cox regression analysis compared to controls (HR = 2.4, p = 0.001), but also to all other IS groups. CLL patients also had the highest rates of positive margins compared to controls (36% vs. 9%, p < 0.01) and to all other IS groups. They were also most likely to get adjuvant or definitive oncological treatments, either radiotherapy or chemotherapy, compared to controls (36% vs. 15%, p = 0.02) and to other IS groups. Patients in the CKD group demonstrated the highest rates for multiple cSCC (OR = 4.7, p = 0.001) and the worst rates of survival on Cox regression analysis (HR = 3.2, p = 0.001). Both rheumatoid arthritis and psoriasis patients demonstrated the shortest disease-free survival rates (2.9y ± 1.1, 2.3y ± 0.7, respectively), compared to controls (4.1y ± 2.8) and to all other IS groups.
Conclusions: Among cSCC patients, immunosuppression due to SOTR, CLL, CKD, RA, and psoriasis is associated with worse outcomes compared to controls and other IS groups. These patients should be regarded as high-risk for developing aggressive cSCC tumors. This study is the first to assess and compare cSCC outcomes among multiple IS patient groups.
Keywords: CLL; chronic kidney disease; cutaneous SCC; immunosuppression; non-melanoma skin cancer; psoriasis; transplants.
Conflict of interest statement
The authors have no ethical conflict to disclose.
Figures

References
-
- Howell J.Y., Ramsey M.L. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Squamous Cell Skin Cancer. - PubMed
-
- Ciążyńska M., Pabianek M., Sławińska M., Reich A., Lewandowski B., Szczepaniak K., Ułańska M., Nejc D., Brodowski R., Sobjanek M., et al. Risk Factors and Clinicopathological Features for Developing a Subsequent Primary Cutaneous Squamous and Basal Cell Carcinomas. Cancers. 2022;14:3069. doi: 10.3390/cancers14133069. - DOI - PMC - PubMed
-
- Schmitt J., Seidler A., Diepgen T.L., Bauer A. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: A systematic review and meta-analysis: Occupational UV exposure and cutaneous SCC. Br. J. Dermatol. 2011;164:291–307. doi: 10.1111/j.1365-2133.2010.10118.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

