Sex Differences in the Systemic and Local Immune Response of Pancreatic Cancer Patients
- PMID: 36980700
- PMCID: PMC10047039
- DOI: 10.3390/cancers15061815
Sex Differences in the Systemic and Local Immune Response of Pancreatic Cancer Patients
Abstract
Background: Mounting evidence suggests that sex plays a critical role in various aspects of cancer such as immune responses. However, a male bias exists in human and non-human studies including immunotherapy trials. The role of sex on immune responses in pancreatic ductal adenocarcinoma (PDA) is unclear.
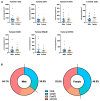
Methods: Here, tumor tissues (tumor and stroma separately) and corresponding blood samples from patients with PDA (n = 52) were systematically analyzed by immunohistochemistry and multiplex cytokine measurements and compared by sex.
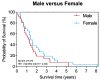
Results: Females showed a stronger systemic immune response with higher levels of CXCL9, IL1B, IL6, IL10 and IL13. Additionally, more peripheral white blood cells were detected in females. In the microenvironment, males showed higher tumoral levels of CXCL12. No differences were detected in the stroma. Females showed a tendency towards an anti-tumoral immune cell profile. CXCL12 blockade indicated a differential microenvironmental effect by sex in an independent immunotherapy trial cohort of patients with PDA (one female, five males). The overall survival did not differ by sex in our cohort.
Conclusion: Systemic and local immune responses differ between sexes in PDA. Accordingly, sex-dependent differences need to be considered in human studies and for specific immunological interventions before clinical translation.
Keywords: CXCL12; pancreatic cancer; pancreatic ductal adenocarcinoma; sex difference; tumor immunology.
Conflict of interest statement
The authors declare no potential competing interests.
Figures



References
-
- Dong M., Cioffi G., Wang J., Waite K.A., Ostrom Q.T., Kruchko C., Lathia J.D., Rubin J.B., Berens M.E., Connor J. Sex Differences in Cancer Incidence and Survival: A Pan-Cancer AnalysisSex Differences in Cancer Incidence and Survival. Cancer Epidemiol. Biomark. Prev. 2020;29:1389–1397. doi: 10.1158/1055-9965.EPI-20-0036. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

