Novel Insights into RAD52's Structure, Function, and Druggability for Synthetic Lethality and Innovative Anticancer Therapies
- PMID: 36980703
- PMCID: PMC10046612
- DOI: 10.3390/cancers15061817
Novel Insights into RAD52's Structure, Function, and Druggability for Synthetic Lethality and Innovative Anticancer Therapies
Abstract
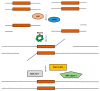
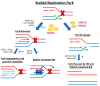
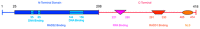
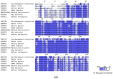
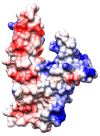
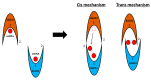
In recent years, the RAD52 protein has been highlighted as a mediator of many DNA repair mechanisms. While RAD52 was initially considered to be a non-essential auxiliary factor, its inhibition has more recently been demonstrated to be synthetically lethal in cancer cells bearing mutations and inactivation of specific intracellular pathways, such as homologous recombination. RAD52 is now recognized as a novel and critical pharmacological target. In this review, we comprehensively describe the available structural and functional information on RAD52. The review highlights the pathways in which RAD52 is involved and the approaches to RAD52 inhibition. We discuss the multifaceted role of this protein, which has a complex, dynamic, and functional 3D superstructural arrangement. This complexity reinforces the need to further investigate and characterize RAD52 to solve a challenging mechanistic puzzle and pave the way for a robust drug discovery campaign.
Keywords: RAD52; drug discovery; precision medicine; synthetic lethality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

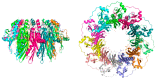

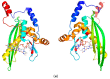
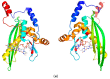
References
-
- Rijkers T., Van Den Ouweland J., Morolli B., Rolink A.G., Baarends W.M., Van Sloun P.P., Lohman P.H., Pastink A. Targeted inactivation of mouse RAD52 reduces homologous recombination but not resistance to ionizing radiation. Mol. Cell. Biol. 1998;18:6423–6429. doi: 10.1128/MCB.18.11.6423. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

