Conditioned Media from Head and Neck Cancer Cell Lines and Serum Samples from Head and Neck Cancer Patients Drive Catabolic Pathways in Cultured Muscle Cells
- PMID: 36980729
- PMCID: PMC10047086
- DOI: 10.3390/cancers15061843
Conditioned Media from Head and Neck Cancer Cell Lines and Serum Samples from Head and Neck Cancer Patients Drive Catabolic Pathways in Cultured Muscle Cells
Abstract
Background: The role of secreted factors from the tumor cells in driving cancer cachexia and especially muscle loss is unknown. We wanted to study both the action of secreted factors from head and neck cancer (HNC) cell lines and circulating factors in HNC patients on skeletal muscle protein catabolism.
Methods: Conditioned media (CM) made from head and neck cancer cell lines and mix of sera from head and neck cancer (HNC) patients were incubated for 48 h with human myotubes. The atrophy and the catabolic pathway were monitored in myotubes. The patients were classified regarding their skeletal muscle loss observed at the outset of management.
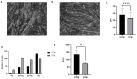
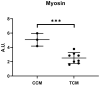
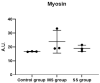
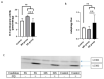
Results: Tumor CM (TCM) was able to produce atrophy on myotubes as compared with control CM (CCM). However, a mix of sera from HNC patients was not able to produce atrophy in myotubes. Despite this discrepancy on atrophy, we observed a similar regulation of the catabolic pathways by the tumor-conditioned media and mix of sera from cancer patients. The catabolic response after incubation with the mix of sera seemed to depend on the muscle loss seen in patients.
Conclusion: This study found evidence that the atrophy observed in HNC patients cannot be solely explained by a deficit in food intake.
Keywords: conditioned media; head and neck cancer; muscle cells protein metabolism; patient serum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Haeggblom L., Attoff T., Yu J., Holzhauser S., Vlastos A., Mirzae L., Ährlund-Richter A., Munck-Wikland E., Marklund L., Hammarstedt-Nordenvall L., et al. Changes in Incidence and Prevalence of Human Papillomavirus in Tonsillar and Base of Tongue Cancer during 2000-2016 in the Stockholm Region and Sweden. Head Neck. 2019;41:1583–1590. doi: 10.1002/hed.25585. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

