Heterochiasmy and Sex Chromosome Evolution in Silene
- PMID: 36980816
- PMCID: PMC10048291
- DOI: 10.3390/genes14030543
Heterochiasmy and Sex Chromosome Evolution in Silene
Abstract
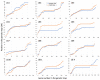
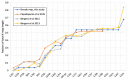
The evolution of a non-recombining sex-specific region is a key step in sex chromosome evolution. Suppression of recombination between the (proto-) X- and Y-chromosomes in male meiosis creates a non-recombining Y-linked region (NRY), while the X-chromosome continues to recombine in females. Lack of recombination in the NRY defines its main properties-genetic degeneration and accumulation of repetitive DNA, making X and Y chromosomes very different from each other. How and why recombination suppression on sex chromosomes evolves remains controversial. A strong difference in recombination rates between the sexes (heterochiasmy) can facilitate or even cause recombination suppression. In the extreme case-complete lack of recombination in the heterogametic sex (achiasmy)-the entire sex-specific chromosome is automatically non-recombining. In this study, I analyse sex-specific recombination rates in a dioecious plant Silene latifolia (Caryophyllaceae), which evolved separate sexes and sex chromosomes ~11 million years ago. I reconstruct high-density RNAseq-based genetic maps including over five thousand genic markers for the two sexes separately. The comparison of the male and female maps reveals only modest heterochiasmy across the genome, with the exception of the sex chromosomes, where recombination is suppressed in males. This indicates that heterochiasmy likely played only a minor, if any, role in NRY evolution in S. latifolia, as recombination suppression is specific to NRY rather than to the entire genome in males. Other mechanisms such as structural rearrangements and/or epigenetic modifications were likely involved, and comparative genome analysis and genetic mapping in multiple Silene species will help to shed light on the mechanism(s) of recombination suppression that led to the evolution of sex chromosomes.
Keywords: Silene latifolia; genetic mapping; heterochiasmy; sex chromosome evolution.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- Ohno S. Sex. Chromosomes and Sex-Linked Genes. Springer; Berlin, Germany: New York, NY, USA: 1967.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

