Therapeutic Targeting of P53: A Comparative Analysis of APR-246 and COTI-2 in Human Tumor Primary Culture 3-D Explants
- PMID: 36981018
- PMCID: PMC10048363
- DOI: 10.3390/genes14030747
Therapeutic Targeting of P53: A Comparative Analysis of APR-246 and COTI-2 in Human Tumor Primary Culture 3-D Explants
Abstract
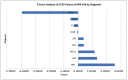
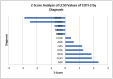
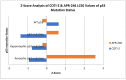
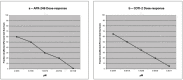
Background:TP53 is the most commonly mutated gene in human cancer with loss of function mutations largely concentrated in "hotspots" affecting DNA binding. APR-246 and COTI-2 are small molecules under investigation in P53 mutated cancers. APR binds to P53 cysteine residues, altering conformation, while COTI-2 showed activity in P53 mutant tumors by a computational platform. We compared APR-246 and COTI-2 activity in human tumor explants from 247 surgical specimens. Methods: Ex vivo analyses of programmed cell death measured drug-induced cell death by delayed-loss-of-membrane integrity and ATP content. The LC50s were compared by Z-Score. Synergy was conducted by the method of Chou and Talalay, and correlations were performed by Pearson moment. Results: APR-246 and COTI-2 activity favored hematologic neoplasms, but solid tumor activity varied by diagnosis. COTI-2 and APR-246 activity did not correlate (R = 0.1028) (NS). COTI-2 activity correlated with nitrogen mustard, cisplatin and gemcitabine, doxorubicin and selumetinib, with a trend for APR-246 with doxorubicin. For ovarian cancer, COTI-2 showed synergy with cisplatin at 25%. Conclusions: COTI-2 and APR-246 activity differ by diagnosis. A lack of correlation supports distinct modes of action. Cisplatin synergy is consistent with P53's role in DNA damage. Different mechanisms of action may underlie disease specificity and offer better disease targeting.
Keywords: APR-246; COTI-2; TP53; anti-cancer drug.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

