Exosomal miRNA Biomarker Panel for Pancreatic Ductal Adenocarcinoma Detection in Patient Plasma: A Pilot Study
- PMID: 36982154
- PMCID: PMC10049393
- DOI: 10.3390/ijms24065081
Exosomal miRNA Biomarker Panel for Pancreatic Ductal Adenocarcinoma Detection in Patient Plasma: A Pilot Study
Abstract
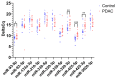
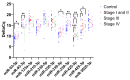
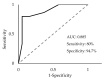
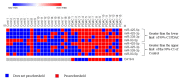
Pancreatic ductal adenocarcinoma (PDAC) is rapidly becoming one of the leading causes of cancer-related deaths in the United States, and with its high mortality rate, there is a pressing need to develop sensitive and robust methods for detection. Exosomal biomarker panels provide a promising avenue for PDAC screening since exosomes are highly stable and easily harvested from body fluids. PDAC-associated miRNAs packaged within these exosomes could be used as diagnostic markers. We analyzed a series of 18 candidate miRNAs via RT-qPCR to identify the differentially expressed miRNAs (p < 0.05, t-test) between plasma exosomes harvested from PDAC patients and control patients. From this analysis, we propose a four-marker panel consisting of miR-93-5p, miR-339-3p, miR-425-5p, and miR-425-3p with an area under the curve (AUC) of the receiver operator characteristic curve (ROC) of 0.885 with a sensitivity of 80% and a specificity of 94.7%, which is comparable to the CA19-9 standard PDAC marker diagnostic.
Keywords: biomarker panel; diagnostics; exosomes; microRNA; pancreatic ducal adenocarcinoma; plasma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

