Intracoronary Imaging of Coronary Atherosclerotic Plaque: From Assessment of Pathophysiological Mechanisms to Therapeutic Implication
- PMID: 36982230
- PMCID: PMC10049285
- DOI: 10.3390/ijms24065155
Intracoronary Imaging of Coronary Atherosclerotic Plaque: From Assessment of Pathophysiological Mechanisms to Therapeutic Implication
Abstract
Atherosclerotic cardiovascular disease is the leading cause of morbidity and mortality worldwide. Several cardiovascular risk factors are implicated in atherosclerotic plaque promotion and progression and are responsible for the clinical manifestations of coronary artery disease (CAD), ranging from chronic to acute coronary syndromes and sudden coronary death. The advent of intravascular imaging (IVI), including intravascular ultrasound, optical coherence tomography and near-infrared diffuse reflectance spectroscopy has significantly improved the comprehension of CAD pathophysiology and has strengthened the prognostic relevance of coronary plaque morphology assessment. Indeed, several atherosclerotic plaque phenotype and mechanisms of plaque destabilization have been recognized with different natural history and prognosis. Finally, IVI demonstrated benefits of secondary prevention therapies, such as lipid-lowering and anti-inflammatory agents. The purpose of this review is to shed light on the principles and properties of available IVI modalities along with their prognostic significance.
Keywords: biological mechanisms; coronary artery disease; intracoronary imaging; plaque healing; plaque vulnerability; secondary prevention therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

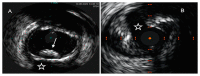
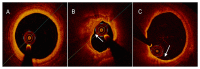
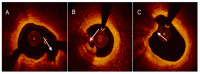
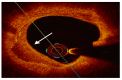



References
-
- Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. Erratum in J. Am. Coll. Cardiol. 2021, 77, 1958–1959. - DOI - PMC - PubMed
-
- Frąk W., Wojtasińska A., Lisińska W., Młynarska E., Franczyk B., Rysz J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines. 2022;10:1938. doi: 10.3390/biomedicines10081938. - DOI - PMC - PubMed
-
- Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., Prescott E., Storey R.F., Deaton C., Cuisset T., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. Erratum in Eur. Heart J. 2020, 41, 4242. - DOI - PubMed
-
- Collet J.-P., Thiele H., Barbato E., Barthélémy O., Bauersachs J., Bhatt D.L., Dendale P., Dorobantu M., Edvardsen T., Folliguet T., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. Erratum in Eur. Heart J. 2021, 42, 1908; Erratum in Eur. Heart J. 2021, 42, 1925; Erratum in Eur. Heart J. 2021. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

