Quantum-Chemical Prediction of Molecular and Electronic Structure of Carbon-Nitrogen Chemical Compound with Unusual Ratio Atoms: C(N20)
- PMID: 36982246
- PMCID: PMC10049734
- DOI: 10.3390/ijms24065172
Quantum-Chemical Prediction of Molecular and Electronic Structure of Carbon-Nitrogen Chemical Compound with Unusual Ratio Atoms: C(N20)
Abstract
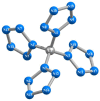
Using various versions of quantum-chemical calculation, namely four versions of density functional theory (DFT), (DFT B3PW91/TZVP, DFT M06/TZVP, DFT B3PW91/Def2TZVP, and DFT M06/Def2TZVP) and two versions of the MP method (MP2/TZVP and MP3/TZVP), the existence possibility of the carbon-nitrogen-containing compound having an unusual M: nitrogen ratio of 1:20, unknown for these elements at present, was shown. Structural parameters data are presented; it was noted that, as may be expected, CN4 grouping has practically a tetrahedral structure, and the chemical bond lengths formed by nitrogen atoms and a carbon atom in the frameworks of each of the calculation methods indicated above are equal to each other. Thermodynamical parameters, NBO analysis data, and HOMO/LUMO images for this compound are also presented. A good agreement between the calculated data obtained using the above three quantum-chemical methods was noticed, too.
Keywords: C(N20); carbon-nitrogen compound; molecular structure; quantum-chemical calculation method.
Conflict of interest statement
The authors declare that they have no conflict of interest, financial or otherwise.
Figures
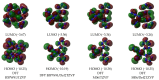
Similar articles
-
Twelve-Nitrogen-Atom Cyclic Structure Stabilized by 3d-Element Atoms: Quantum Chemical Modeling.Int J Mol Sci. 2022 Jun 12;23(12):6560. doi: 10.3390/ijms23126560. Int J Mol Sci. 2022. PMID: 35743004 Free PMC article.
-
A new chemical compound with an unusual ratio of number of carbon and nitrogen atoms - C(N12): quantum-chemical modelling.RSC Adv. 2021 Nov 8;11(57):35974-35981. doi: 10.1039/d1ra07549g. eCollection 2021 Nov 4. RSC Adv. 2021. PMID: 35492795 Free PMC article.
-
Cubic Octa-Carbon: Quantum-Chemical Design of Molecular Structure and Potential Way of Its Synthesis from Cubane.Int J Mol Sci. 2021 Nov 8;22(21):12067. doi: 10.3390/ijms222112067. Int J Mol Sci. 2021. PMID: 34769494 Free PMC article.
-
First Examples of s-Metal Complexes with Subporphyrazine and Its Phenylene-Annulated Derivatives: DFT Calculations.Int J Mol Sci. 2024 Jun 24;25(13):6897. doi: 10.3390/ijms25136897. Int J Mol Sci. 2024. PMID: 39000007 Free PMC article.
-
Molecular and Electronic Structures of Neutral Polynitrogens: Review on the Theory and Experiment in 21st Century.Int J Mol Sci. 2022 Mar 4;23(5):2841. doi: 10.3390/ijms23052841. Int J Mol Sci. 2022. PMID: 35269983 Free PMC article. Review.
References
-
- Ringer A.L., Sherrill C.D., King R.A., Crawford T.D. Low-lying singlet excited states of isocyanogen. Int. J. Quantum Chem. 2008;106:1137–1140. doi: 10.1002/qua.21586. - DOI
-
- Brotherton T.K., Lynn J.W. The Synthesis and Chemistry of Cyanogen. Chem. Revs. 1959;59:841–883. doi: 10.1021/cr50029a003. - DOI
-
- Bircumshaw L.L., Tayler F.M., Whiffen D.H. Paracyanogen: Its formation and properties. Part I. J. Chem. Soc. 1954:931–935. doi: 10.1039/jr9540000931. - DOI
-
- Franklin E.C. The Ammono Carbonic Acids. J. Am. Chem. Soc. 1922;44:486–509. doi: 10.1021/ja01424a007. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

