Dual Inoculation with Rhizophagus irregularis and Bacillus megaterium Improves Maize Tolerance to Combined Drought and High Temperature Stress by Enhancing Root Hydraulics, Photosynthesis and Hormonal Responses
- PMID: 36982272
- PMCID: PMC10049376
- DOI: 10.3390/ijms24065193
Dual Inoculation with Rhizophagus irregularis and Bacillus megaterium Improves Maize Tolerance to Combined Drought and High Temperature Stress by Enhancing Root Hydraulics, Photosynthesis and Hormonal Responses
Abstract
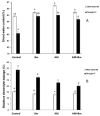
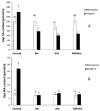
Climate change is leading to combined drought and high temperature stress in many areas, drastically reducing crop production, especially for high-water-consuming crops such as maize. This study aimed to determine how the co-inoculation of an arbuscular mycorrhizal (AM) fungus (Rhizophagus irregularis) and the PGPR Bacillus megaterium (Bm) alters the radial water movement and physiology in maize plants in order to cope with combined drought and high temperature stress. Thus, maize plants were kept uninoculated or inoculated with R. irregularis (AM), with B. megaterium (Bm) or with both microorganisms (AM + Bm) and subjected or not to combined drought and high temperature stress (D + T). We measured plant physiological responses, root hydraulic parameters, aquaporin gene expression and protein abundances and sap hormonal content. The results showed that dual AM + Bm inoculation was more effective against combined D + T stress than single inoculation. This was related to a synergistic enhancement of efficiency of the phytosystem II, stomatal conductance and photosynthetic activity. Moreover, dually inoculated plants maintained higher root hydraulic conductivity, which was related to regulation of the aquaporins ZmPIP1;3, ZmTIP1.1, ZmPIP2;2 and GintAQPF1 and levels of plant sap hormones. This study demonstrates the usefulness of combining beneficial soil microorganisms to improve crop productivity under the current climate-change scenario.
Keywords: PGPR; aquaporin; arbuscular mycorrhiza; combined drought and heat stress; maize; root hydraulic conductivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Trenberth K.E., Dai A., Van der Schrier G., Jones P.D., Barichivich J., Briffa K.R., Sheffield J. Global warming and changes in drought. Nat. Clim. Chang. 2014;4:17–22. doi: 10.1038/nclimate2067. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

