Pentacoordinated Organotin(IV) Complexes as an Alternative in the Design of Highly Efficient Optoelectronic and Photovoltaic Devices: Synthesis and Photophysical Characterization
- PMID: 36982325
- PMCID: PMC10049675
- DOI: 10.3390/ijms24065255
Pentacoordinated Organotin(IV) Complexes as an Alternative in the Design of Highly Efficient Optoelectronic and Photovoltaic Devices: Synthesis and Photophysical Characterization
Abstract
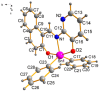
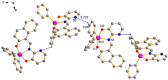
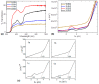
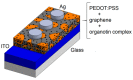
The synthesis of four pentacoordinated organotin(IV) complexes prepared in a one-pot reaction from 2-hydroxy-1-naphthaldehyde, 2-amino-3-hydroxypyridine and organotin oxides is reported. The complexes were characterized by UV-Vis, IR, MS, 1H, 13C and 119Sn NMR techniques. The compound based on 2,2-diphenyl-6-aza-1,3-dioxa-2-stannanaphtho[1,2-h]pyrido[3,2-d]cyclononene revealed the formation of a monomeric complex with a distorted five-coordinated molecular geometry intermediate between the trigonal bipyramidal and square pyramidal. In order to find possible applications in photovoltaic devices, hybrid films of organotin(IV) complexes embedded in poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) with graphene were deposited. The topographic and mechanical properties were examined. The film with the complex integrated into the cyclohexyl substituent has high plastic deformation, with a maximum stress of 1.69 × 107 Pa and a Knoop hardness of 0.061. The lowest values of 1.85 eV for the onset gap and 3.53 eV for the energy gap were obtained for the heterostructure having the complex with the phenyl substituent. Bulk heterojunction devices were fabricated; these devices showed ohmic behavior at low voltages and a space-charge-limited current (SCLC) conduction mechanism at higher voltages. A value of 0.02 A was found for the maximum carried current. The SCLC mechanism suggests hole mobility values of between 2.62 × 10-2 and 3.63 cm2/V.s and concentrations of thermally excited holes between 2.96 × 1018 and 4.38 × 1018 m-3.
Keywords: PEDOT:PSS; electrical properties; graphene; hybrid film; optical properties; organotin(IV) complexes; organotin–Schiff bases; pentacoordinated organotin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Yuan J., Ford M.J., Xu Y., Zhang Y., Bazan G.C., Ma W. Improved Tandem All-Polymer Solar Cells Performance by Using Spectrally Matched Subcells. Adv. Energy Mater. 2018;8:1703291. doi: 10.1002/aenm.201703291. - DOI
-
- Qin P., Zhang J., Yang G., Yu X., Li G. Potassium-intercalated rubrene as a dual-functional passivation agent for high efficiency perovskite solar cells. J. Mater. Chem. A. 2018;7:1824–1834. doi: 10.1039/C8TA09026B. - DOI
-
- Duan T., Tang H., Liang R.-Z., Lv J., Kan Z., Singh R., Kumar M., Xiao Z., Lu S., Laquai F. Terminal group engineering for small-molecule donors boosts the performance of nonfullerene organic solar cells. J. Mater. Chem. A. 2019;7:2541–2546. doi: 10.1039/C8TA11420J. - DOI
-
- Zhai Z., Xu M. All-solution-processed small-molecule solar cells by stripping-transfer method. J. Mater. Sci. Mater. Electron. 2020;31:5789–5793. doi: 10.1007/s10854-020-03149-5. - DOI
-
- Collins S.D., Ran N.A., Heiber M.C., Nguyen T.-Q. Small is Powerful: Recent Progress in Solution-Processed Small Molecule Solar Cells. Adv. Energy Mater. 2017;7:1602242. doi: 10.1002/aenm.201602242. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

