KDM5D Histone Demethylase Identifies Platinum-Tolerant Head and Neck Cancer Cells Vulnerable to Mitotic Catastrophe
- PMID: 36982384
- PMCID: PMC10049674
- DOI: 10.3390/ijms24065310
KDM5D Histone Demethylase Identifies Platinum-Tolerant Head and Neck Cancer Cells Vulnerable to Mitotic Catastrophe
Abstract
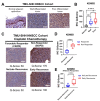
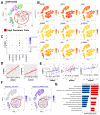
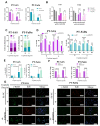
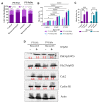
Head and neck squamous cell carcinoma (HNSCC) is a major contributor to cancer incidence globally and is currently managed by surgical resection followed by adjuvant chemoradiotherapy. However, local recurrence is the major cause of mortality, indicating the emergence of drug-tolerant persister cells. A specific histone demethylase, namely lysine-specific demethylase 5D (KDM5D), is overexpressed in diverse types of cancers and involved in cancer cell cycle regulation. However, the role of KDM5D in the development of cisplatin-tolerant persister cells remains unexplored. Here, we demonstrated that KDM5D contributes to the development of persister cells. Aurora Kinase B (AURKB) disruption affected the vulnerability of persister cells in a mitotic catastrophe-dependent manner. Comprehensive in silico, in vitro, and in vivo experiments were performed. KDM5D expression was upregulated in HNSCC tumor cells, cancer stem cells, and cisplatin-resistant cells with biologically distinct signaling alterations. In an HNSCC cohort, high KDM5D expression was associated with a poor response to platinum treatment and early disease recurrence. KDM5D knockdown reduced the tolerance of persister cells to platinum agents and caused marked cell cycle deregulation, including the loss of DNA damage prevention, and abnormal mitosis-enhanced cell cycle arrest. By modulating mRNA levels of AURKB, KDM5D promoted the generation of platinum-tolerant persister cells in vitro, leading to the identification of the KDM5D/AURKB axis, which regulates cancer stemness and drug tolerance of HNSCC. Treatment with an AURKB inhibitor, namely barasertib, resulted in a lethal consequence of mitotic catastrophe in HNSCC persister cells. The cotreatment of cisplatin and barasertib suppressed tumor growth in the tumor mouse model. Thus, KDM5D might be involved in the development of persister cells, and AURKB disruption can overcome tolerance to platinum treatment in HNSCC.
Keywords: AURKB; KDM5D; cisplatin; drug-tolerant persister; head and neck cancer.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors stated they have not any potential financial competing interests that could in any way gain or lose financially in the publication of this manuscript now or the future. Furthermore, not any nonfinancial competing interests are involved in the manuscript.
Figures



References
-
- Burtness B., Harrington K.J., Greil R., Soulières D., Tahara M., de Castro G., Jr. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

