New Pathways Identify Novel Drug Targets for the Prevention and Treatment of Alzheimer's Disease
- PMID: 36982456
- PMCID: PMC10049476
- DOI: 10.3390/ijms24065383
New Pathways Identify Novel Drug Targets for the Prevention and Treatment of Alzheimer's Disease
Abstract
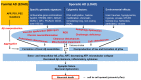
Alzheimer's disease (AD) is an incurable, progressive neurodegenerative disorder. AD is a complex and multifactorial disease that is responsible for 60-80% of dementia cases. Aging, genetic factors, and epigenetic changes are the main risk factors for AD. Two aggregation-prone proteins play a decisive role in AD pathogenesis: β-amyloid (Aβ) and hyperphosphorylated tau (pTau). Both of them form deposits and diffusible toxic aggregates in the brain. These proteins are the biomarkers of AD. Different hypotheses have tried to explain AD pathogenesis and served as platforms for AD drug research. Experiments demonstrated that both Aβ and pTau might start neurodegenerative processes and are necessary for cognitive decline. The two pathologies act in synergy. Inhibition of the formation of toxic Aβ and pTau aggregates has been an old drug target. Recently, successful Aβ clearance by monoclonal antibodies has raised new hopes for AD treatments if the disease is detected at early stages. More recently, novel targets, e.g., improvements in amyloid clearance from the brain, application of small heat shock proteins (Hsps), modulation of chronic neuroinflammation by different receptor ligands, modulation of microglial phagocytosis, and increase in myelination have been revealed in AD research.
Keywords: Alzheimer’s disease; Aβ; amyloid clearance; drug targets; genetics; heat shock proteins; neuroinflammation; tau; toxic amyloids; vascular dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

