Benzo(a)pyrene and Cerium Dioxide Nanoparticles in Co-Exposure Impair Human Trophoblast Cell Stress Signaling
- PMID: 36982514
- PMCID: PMC10049531
- DOI: 10.3390/ijms24065439
Benzo(a)pyrene and Cerium Dioxide Nanoparticles in Co-Exposure Impair Human Trophoblast Cell Stress Signaling
Abstract
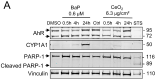
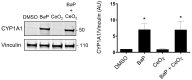
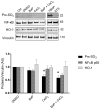
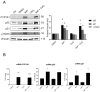
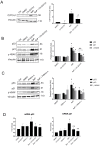
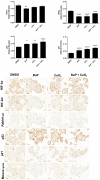
Human placenta is a multifunctional interface between maternal and fetal blood. Studying the impact of pollutants on this organ is crucial because many xenobiotics in maternal blood can accumulate in placental cells or pass into the fetal circulation. Benzo(a)pyrene (BaP) and cerium dioxide nanoparticles (CeO2 NP), which share the same emission sources, are found in ambient air pollution and also in maternal blood. The aim of the study was to depict the main signaling pathways modulated after exposure to BaP or CeO2 NP vs. co-exposure on both chorionic villi explants and villous cytotrophoblasts isolated from human term placenta. At nontoxic doses of pollutants, BaP is bioactivated by AhR xenobiotic metabolizing enzymes, leading to DNA damage with an increase in γ-H2AX, the stabilization of stress transcription factor p53, and the induction of its target p21. These effects are reproduced in co-exposure with CeO2 NP, except for the increase in γ-H2AX, which suggests a modulation of the genotoxic effect of BaP by CeO2 NP. Moreover, CeO2 NP in individual and co-exposure lead to a decrease in Prx-SO3, suggesting an antioxidant effect. This study is the first to identify the signaling pathways modulated after co-exposure to these two pollutants, which are common in the environment.
Keywords: AhR; CYP1A1; benzo(a)pyrene; cerium dioxide nanoparticles; chorionic villi; human placenta; p21; p53; villous cytotrophoblasts; γ-H2AX.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Saenen N.D., Martens D.S., Neven K.Y., Alfano R., Bové H., Janssen B.G., Roels H.A., Plusquin M., Vrijens K., Nawrot T.S. Air pollution-induced placental alterations: An interplay of oxidative stress, epigenetics, and the aging phenotype? Clin. Epigenetics. 2019;11:124. doi: 10.1186/s13148-019-0688-z. - DOI - PMC - PubMed
-
- Wakx A., Nedder M., Tomkiewicz-Raulet C., Dalmasso J., Chissey A., Boland S., Vibert F., Degrelle S.A., Fournier T., Coumoul X., et al. Expression, Localization, and Activity of the Aryl Hydrocarbon Receptor in the Human Placenta. Int. J. Mol. Sci. 2018;19:3762. doi: 10.3390/ijms19123762. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

