IL-1 Mediates Chronic Stress-Induced Hyperalgesia Accompanied by Microglia and Astroglia Morphological Changes in Pain-Related Brain Regions in Mice
- PMID: 36982563
- PMCID: PMC10052634
- DOI: 10.3390/ijms24065479
IL-1 Mediates Chronic Stress-Induced Hyperalgesia Accompanied by Microglia and Astroglia Morphological Changes in Pain-Related Brain Regions in Mice
Abstract
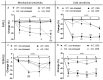
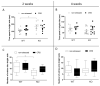
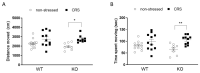
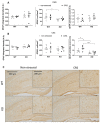
Chronic stress causes several pain conditions including fibromyalgia. Its pathophysiological mechanisms are unknown, and the therapy is unresolved. Since the involvement of interleukin-1 (IL-1) has been described in stress and inflammatory pain but no data are available regarding stress-induced pain, we studied its role in a chronic restraint stress (CRS) mouse model. Female and male C57Bl/6J wild-type (WT) and IL-1αβ-deficient (knock-out: IL-1 KO) mice were exposed to 6 h of immobilization/day for 4 weeks. Mechanonociception, cold tolerance, behavioral alterations, relative thymus/adrenal gland weights, microglia ionized calcium-binding adaptor molecule 1 (IBA1) and astrocyte glial fibrillary acidic protein (GFAP) integrated density, number and morphological transformation in pain-related brain regions were determined. CRS induced 15-20% mechanical hyperalgesia after 2 weeks in WT mice in both sexes, which was significantly reduced in female but not in male IL-1 KOs. Increased IBA1+ integrated density in the central nucleus of amygdala, primary somatosensory cortex hind limb representation part, hippocampus cornu ammonis area 3 (CA3) and periaqueductal gray matter (PAG) was present, accompanied by a cell number increase in IBA1+ microglia in stressed female WTs but not in IL-1 KOs. CRS induced morphological changes of GFAP+ astrocytes in WT but not in KO mice. Stress evoked cold hypersensitivity in the stressed animals. Anxiety and depression-like behaviors, thymus and adrenal gland weight changes were detectable in all groups after 2 but not 4 weeks of CRS due to adaptation. Thus, IL-1 mediates chronic stress-induced hyperalgesia in female mice, without other major behavioral alterations, suggesting the analgesic potentials of IL-1 in blocking drugs in stress-related pain syndromes.
Keywords: central pain sensitization; chronic immobilization; cytokines; fibromyalgia-like pain syndrome; hyperalgesia; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- National Institute for Health and Care Excellence . NICE Chronic Pain (Primary and Secondary) in over 16s: Assessment of All Chronic Pain and Management of Chronic Primary Pain. National Institute for Health and Care Excellence (NICE); London, UK: 2021. - PubMed
-
- Vincent A., Lahr B.D., Wolfe F., Clauw D.J., Whipple M.O., Oh T.H., Barton D.L., St. Sauver J. Prevalence of Fibromyalgia: A Population-Based Study in Olmsted County, Minnesota, Utilizing the Rochester Epidemiology Project. Arthritis Care Res. 2013;65:786–792. doi: 10.1002/acr.21896. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- (BO/00592/19/5)/János Bolyai Research Scholarship of the Hungarian Academy of Sciences
- UNKP-25-5-PTE-1447/ÚNKP-21-5 new National Excellence Program of the Ministry for Innovation and Technology
- TKP2021-EGA-16 TKP2021-EGA-13/National Research, Development and Innovation Fund of Hungary, financed under the EGA 16 funding scheme
- OTKA K138046, OTKA FK137951/National Brain Research Program 3.0, National Research, Development and Innovation Office
- NA/Eötvös Loránd Research Network (Chronic Pain Research Group), Pécs
- Project no. RRF-2.3.1-21-2022-00011, RRF-2.3.1-21-2022-00015/National Laboratory of Translational Neuroscience has been implemented with the support provided by the Recovery and Resilience Facility of the Europian Union within the framework of Programme Széchenyi Plan Plus
- (BO/00750/22/5)/V.K. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences
- (UNKP-22-5-PTE-1740)/The New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund
- (KA-2022-29)/The Research grant of Medical School, University of Pécs
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

