Synergistic Interaction of the Class IIa HDAC Inhibitor CHDI0039 with Bortezomib in Head and Neck Cancer Cells
- PMID: 36982651
- PMCID: PMC10056166
- DOI: 10.3390/ijms24065553
Synergistic Interaction of the Class IIa HDAC Inhibitor CHDI0039 with Bortezomib in Head and Neck Cancer Cells
Abstract
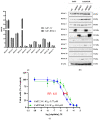
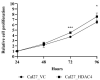
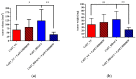
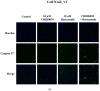
In contrast to class I/IIb/pan histone deacetylase inhibitors (HDACi), the role of class IIa HDACi as anti-cancer chemosensitizing agents is less well understood. Here, we studied the effects of HDAC4 in particular and the class IIa HDACi CHDI0039 on proliferation and chemosensitivity in Cal27 and cisplatin-resistant Cal27CisR head and neck squamous cell cancer (HNSCC). HDAC4 and HDAC5 overexpression clones were generated. HDAC4 overexpression (Cal27_HDAC4) increased proliferation significantly compared to vector control cells (Cal27_VC). Chicken chorioallantoic membrane (CAM) studies confirmed the in vitro results: Cal27_HDAC4 tumors were slightly larger than tumors from Cal27_VC, and treatment with CHDI0039 resulted in a significant decrease in tumor size and weight of Cal27_HDAC4 but not Cal27_VC. Unlike class I/pan-HDACi, treatment with CHDI0039 had only a marginal impact on cisplatin cytotoxicity irrespective of HDAC4 and HDAC5 expression. In contrast, the combination of CHDI0039 with bortezomib was synergistic (Chou-Talalay) in MTT and caspase 3/7 activation experiments. RNAseq indicated that treatment with CHDI0039 alters the expression of genes whose up- or downregulation is associated with increased survival in HNSCC patients according to Kaplan-Meier data. We conclude that the combination of class IIa HDACi with proteasome inhibitors constitutes an effective treatment option for HNSCC, particularly for platinum-resistant cancers.
Keywords: CHDI0039; HDAC inhibitor; HDAC4; HDAC5; bortezomib; class IIa histone deacetylase; head and neck cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

