High-Intensity Training Represses FXYD5 and Glycosylates Na,K-ATPase in Type II Muscle Fibres, Which Are Linked with Improved Muscle K+ Handling and Performance
- PMID: 36982661
- PMCID: PMC10051537
- DOI: 10.3390/ijms24065587
High-Intensity Training Represses FXYD5 and Glycosylates Na,K-ATPase in Type II Muscle Fibres, Which Are Linked with Improved Muscle K+ Handling and Performance
Abstract
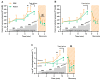
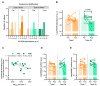
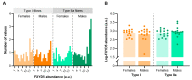
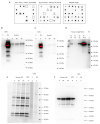
Na+/K+ ATPase (NKA) comprises several subunits to provide isozyme heterogeneity in a tissue-specific manner. An abundance of NKA α, β, and FXYD1 subunits is well-described in human skeletal muscle, but not much is known about FXYD5 (dysadherin), a regulator of NKA and β1 subunit glycosylation, especially with regard to fibre-type specificity and influence of sex and exercise training. Here, we investigated muscle fibre-type specific adaptations in FXYD5 and glycosylated NKAβ1 to high-intensity interval training (HIIT), as well as sex differences in FXYD5 abundance. In nine young males (23.8 ± 2.5 years of age) (mean ± SD), 3 weekly sessions of HIIT for 6 weeks enhanced muscle endurance (220 ± 102 vs. 119 ± 99 s, p < 0.01) and lowered leg K+ release during intense knee-extensor exercise (0.5 ± 0.8 vs. 1.0 ± 0.8 mmol·min-1, p < 0.01) while also increasing cumulated leg K+ reuptake 0-3 min into recovery (2.1 ± 1.5 vs. 0.3 ± 0.9 mmol, p < 0.01). In type IIa muscle fibres, HIIT lowered FXYD5 abundance (p < 0.01) and increased the relative distribution of glycosylated NKAβ1 (p < 0.05). FXYD5 abundance in type IIa muscle fibres correlated inversely with the maximal oxygen consumption (r = -0.53, p < 0.05). NKAα2 and β1 subunit abundances did not change with HIIT. In muscle fibres from 30 trained males and females, we observed no sex (p = 0.87) or fibre type differences (p = 0.44) in FXYD5 abundance. Thus, HIIT downregulates FXYD5 and increases the distribution of glycosylated NKAβ1 in type IIa muscle fibres, which is likely independent of a change in the number of NKA complexes. These adaptations may contribute to counter exercise-related K+ shifts and enhance muscle performance during intense exercise.
Keywords: ATPase; FXYD; NKA; Na-K; beta; dysadherin; isoform; phospholemman; pump; subunit.
Conflict of interest statement
The authors declare no conflict of interest related to the present work.
Figures

Similar articles
-
Effect of differentiation, de novo innervation, and electrical pulse stimulation on mRNA and protein expression of Na+,K+-ATPase, FXYD1, and FXYD5 in cultured human skeletal muscle cells.PLoS One. 2021 Feb 26;16(2):e0247377. doi: 10.1371/journal.pone.0247377. eCollection 2021. PLoS One. 2021. PMID: 33635930 Free PMC article.
-
Cycling with blood flow restriction improves performance and muscle K+ regulation and alters the effect of anti-oxidant infusion in humans.J Physiol. 2019 May;597(9):2421-2444. doi: 10.1113/JP277657. Epub 2019 Mar 28. J Physiol. 2019. PMID: 30843602 Free PMC article.
-
Single-fiber expression and fiber-specific adaptability to short-term intense exercise training of Na+-K+-ATPase α- and β-isoforms in human skeletal muscle.J Appl Physiol (1985). 2015 Mar 15;118(6):699-706. doi: 10.1152/japplphysiol.00419.2014. Epub 2015 Jan 22. J Appl Physiol (1985). 2015. PMID: 25614596
-
Inactivity and exercise training differentially regulate abundance of Na+-K+-ATPase in human skeletal muscle.J Appl Physiol (1985). 2019 Oct 1;127(4):905-920. doi: 10.1152/japplphysiol.01076.2018. Epub 2019 Aug 1. J Appl Physiol (1985). 2019. PMID: 31369327 Review.
-
A century of exercise physiology: effects of muscle contraction and exercise on skeletal muscle Na+,K+-ATPase, Na+ and K+ ions, and on plasma K+ concentration-historical developments.Eur J Appl Physiol. 2024 Mar;124(3):681-751. doi: 10.1007/s00421-023-05335-9. Epub 2024 Jan 11. Eur J Appl Physiol. 2024. PMID: 38206444 Free PMC article. Review.
Cited by
-
AMPK and glucose deprivation exert an isoform-specific effect on the expression of Na+,K+-ATPase subunits in cultured myotubes.J Muscle Res Cell Motil. 2024 Sep;45(3):139-154. doi: 10.1007/s10974-024-09673-9. Epub 2024 May 6. J Muscle Res Cell Motil. 2024. PMID: 38709429 Free PMC article.
-
The mitochondrial mRNA-stabilizing protein SLIRP regulates skeletal muscle mitochondrial structure and respiration by exercise-recoverable mechanisms.Nat Commun. 2024 Nov 13;15(1):9826. doi: 10.1038/s41467-024-54183-4. Nat Commun. 2024. PMID: 39537626 Free PMC article.
-
Evaluating the impact of coenzyme Q10 and high-intensity interval training on lactate threshold and Plasma blood gases in rats: a randomized controlled trial.Eur J Appl Physiol. 2025 Aug;125(8):2185-2196. doi: 10.1007/s00421-025-05756-8. Epub 2025 Mar 18. Eur J Appl Physiol. 2025. PMID: 40100404 Free PMC article.
-
Fiber Type-Specific Adaptations to Exercise Training in Human Skeletal Muscle: Lessons From Proteome Analyses and Future Directions.Scand J Med Sci Sports. 2025 May;35(5):e70059. doi: 10.1111/sms.70059. Scand J Med Sci Sports. 2025. PMID: 40281372 Free PMC article. Review.
-
"Cardiac glycosides"-quo vaditis?-past, present, and future?Naunyn Schmiedebergs Arch Pharmacol. 2024 Dec;397(12):9521-9531. doi: 10.1007/s00210-024-03285-3. Epub 2024 Jul 15. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39007928 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

