Dopamine Transmission Imbalance in Neuroinflammation: Perspectives on Long-Term COVID-19
- PMID: 36982693
- PMCID: PMC10056044
- DOI: 10.3390/ijms24065618
Dopamine Transmission Imbalance in Neuroinflammation: Perspectives on Long-Term COVID-19
Abstract
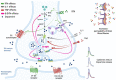
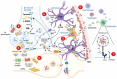
Dopamine (DA) is a key neurotransmitter in the basal ganglia, implicated in the control of movement and motivation. Alteration of DA levels is central in Parkinson's disease (PD), a common neurodegenerative disorder characterized by motor and non-motor manifestations and deposition of alpha-synuclein (α-syn) aggregates. Previous studies have hypothesized a link between PD and viral infections. Indeed, different cases of parkinsonism have been reported following COVID-19. However, whether SARS-CoV-2 may trigger a neurodegenerative process is still a matter of debate. Interestingly, evidence of brain inflammation has been described in postmortem samples of patients infected by SARS-CoV-2, which suggests immune-mediated mechanisms triggering the neurological sequelae. In this review, we discuss the role of proinflammatory molecules such as cytokines, chemokines, and oxygen reactive species in modulating DA homeostasis. Moreover, we review the existing literature on the possible mechanistic interplay between SARS-CoV-2-mediated neuroinflammation and nigrostriatal DAergic impairment, and the cross-talk with aberrant α-syn metabolism.
Keywords: Parkinson’s disease; SARS-CoV-2; alpha-synuclein; cytokines; dopamine; dopamine release; glia; interleukins; long-COVID; neuroinflammation; post-acute sequelae.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

