Functional and Phenotypic Characterisations of Common Syngeneic Tumour Cell Lines as Estrogen Receptor-Positive Breast Cancer Models
- PMID: 36982737
- PMCID: PMC10053941
- DOI: 10.3390/ijms24065666
Functional and Phenotypic Characterisations of Common Syngeneic Tumour Cell Lines as Estrogen Receptor-Positive Breast Cancer Models
Abstract
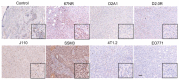
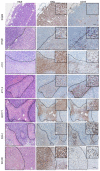
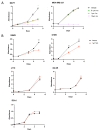
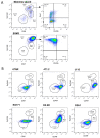
Estrogen receptor-positive breast cancers (ER+ BCas) are the most common form of BCa and are increasing in incidence, largely due to changes in reproductive practices in recent decades. Tamoxifen is prescribed as a component of standard-of-care endocrine therapy for the treatment and prevention of ER+ BCa. However, it is poorly tolerated, leading to low uptake of the drug in the preventative setting. Alternative therapies and preventatives for ER+ BCa are needed but development is hampered due to a paucity of syngeneic ER+ preclinical mouse models that allow pre-clinical experimentation in immunocompetent mice. Two ER-positive models, J110 and SSM3, have been reported in addition to other tumour models occasionally shown to express ER (for example 4T1.2, 67NR, EO771, D2.0R and D2A1). Here, we have assessed ER expression and protein levels in seven mouse mammary tumour cell lines and their corresponding tumours, in addition to their cellular composition, tamoxifen sensitivity and molecular phenotype. By immunohistochemical assessment, SSM3 and, to a lesser extent, 67NR cells are ER+. Using flow cytometry and transcript expression we show that SSM3 cells are luminal in nature, whilst D2.0R and J110 cells are stromal/basal. The remainder are also stromal/basal in nature; displaying a stromal or basal Epcam/CD49f FACS phenotype and stromal and basal gene expression signatures are overrepresented in their transcript profile. Consistent with a luminal identity for SSM3 cells, they also show sensitivity to tamoxifen in vitro and in vivo. In conclusion, the data indicate that the SSM3 syngeneic cell line is the only definitively ER+ mouse mammary tumour cell line widely available for pre-clinical research.
Keywords: breast cancer cell line; estrogen receptor-positive; mouse models; syngeneic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cuzick J., Sestak I., Cawthorn S., Hamed H., Holli K., Howell A., Forbes J.F., Investigators I.-I. Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16:67–75. doi: 10.1016/S1470-2045(14)71171-4. - DOI - PMC - PubMed
-
- Donnelly L., Evans D., Wiseman J., Fox J., Greenhalgh R., Affen J., Juraskova I., Stavrinos P., Dawe S., Cuzick J. Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic. Br. J. Cancer. 2014;110:1681–1687. doi: 10.1038/bjc.2014.109. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

