CAR T-Cell Persistence Correlates with Improved Outcome in Patients with B-Cell Lymphoma
- PMID: 36982764
- PMCID: PMC10056741
- DOI: 10.3390/ijms24065688
CAR T-Cell Persistence Correlates with Improved Outcome in Patients with B-Cell Lymphoma
Abstract
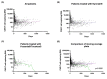
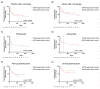
Chimeric antigen receptor (CAR) T-cell therapy has led to profound and durable tumor responses in a relevant subset of patients with relapsed/refractory (r/r) B-cell lymphomas. Still, some patients show insufficient benefit or relapse after CAR T-cell therapy. We performed a retrospective study to investigate the correlation between CAR T-cell persistence in the peripheral blood (PB) at 6 months, assessed by droplet digital PCR (ddPCR), with CAR T-cell treatment outcome. 92 patients with r/r B-cell lymphomas were treated with CD19-targeting CAR T-cell therapies at our institution between 01/2019-08/2022. Six months post-treatment, 15 (16%) patients had no detectable circulating CAR-T constructs by ddPCR. Patients with CAR T-cell persistence had a significantly higher CAR T-cell peak (5432 vs. 620 copies/ug cfDNA, p = 0.0096), as well as higher incidence of immune effector cell-associated neurotoxicity syndrome (37% vs. 7%, p = 0.0182). After a median follow-up of 8.5 months, 31 (34%) patients relapsed. Lymphoma relapses were less frequent among patients with CAR T-cell persistence (29% vs. 60%, p = 0.0336), and CAR T-cell persistence in the PB at 6 months was associated with longer progression-free survival (PFS) (HR 2.79, 95% CI: 1.09-7.11, p = 0.0319). Moreover, we observed a trend towards improved overall survival (OS) (HR 1.99, 95% CI: 0.68-5.82, p = 0.2092) for these patients. In our cohort of 92 B-cell lymphomas, CAR T-cell persistence at 6 months was associated with lower relapse rates and longer PFS. Moreover, our data confirm that 4-1BB-CAR T-cells have a longer persistence as compared to CD-28-based CAR T-cells.
Keywords: B-cell lymphoma; CAR T-cell persistence; CAR T-cell therapy; ddPCR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Vercellino L., Di Blasi R., Kanoun S., Tessoulin B., Rossi C., D’Aveni-Piney M., Obéric L., Bodet-Milin C., Bories P., Olivier P., et al. Predictive Factors of Early Progression after CAR T-Cell Therapy in Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood Adv. 2020;4:5607–5615. doi: 10.1182/bloodadvances.2020003001. - DOI - PMC - PubMed
-
- Nakaya A., Fujita S., Satake A., Nakanishi T., Azuma Y., Tsubokura Y., Hotta M., Yoshimura H., Ishii K., Ito T., et al. Upfront High-Dose Chemotherapy Combined with Autologous Stem Cell Transplantation: Potential Survival Benefit for Patients with High-Risk Diffuse Large B-Cell Lymphoma. Oncol. Lett. 2017;14:3803–3808. doi: 10.3892/ol.2017.6589. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

