Adipose-Derived Stem Cells in Reinforced Collagen Gel: A Comparison between Two Approaches to Differentiation towards Smooth Muscle Cells
- PMID: 36982766
- PMCID: PMC10058441
- DOI: 10.3390/ijms24065692
Adipose-Derived Stem Cells in Reinforced Collagen Gel: A Comparison between Two Approaches to Differentiation towards Smooth Muscle Cells
Abstract
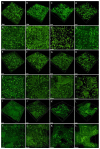
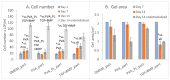
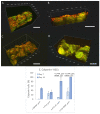
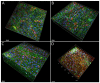
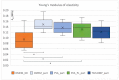
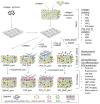
Scaffolds made of degradable polymers, such as collagen, polyesters or polysaccharides, are promising matrices for fabrication of bioartificial vascular grafts or patches. In this study, collagen isolated from porcine skin was processed into a gel, reinforced with collagen particles and with incorporated adipose tissue-derived stem cells (ASCs). The cell-material constructs were then incubated in a DMEM medium with 2% of FS (DMEM_part), with added polyvinylalcohol nanofibers (PVA_part sample), and for ASCs differentiation towards smooth muscle cells (SMCs), the medium was supplemented either with human platelet lysate released from PVA nanofibers (PVA_PL_part) or with TGF-β1 + BMP-4 (TGF + BMP_part). The constructs were further endothelialised with human umbilical vein endothelial cells (ECs). The immunofluorescence staining of alpha-actin and calponin, and von Willebrand factor, was performed. The proteins involved in cell differentiation, the extracellular matrix (ECM) proteins, and ECM remodelling proteins were evaluated by mass spectrometry on day 12 of culture. Mechanical properties of the gels with ASCs were measured via an unconfined compression test on day 5. Gels evinced limited planar shrinkage, but it was higher in endothelialised TGF + BMP_part gel. Both PVA_PL_part samples and TGF + BMP_part samples supported ASC growth and differentiation towards SMCs, but only PVA_PL_part supported homogeneous endothelialisation. Young modulus of elasticity increased in all samples compared to day 0, and PVA_PL_part gel evinced a slightly higher ratio of elastic energy. The results suggest that PVA_PL_part collagen construct has the highest potential to remodel into a functional vascular wall.
Keywords: adipose tissue-derived stem cells; collagen particles; endothelial cells; extracellular matrix; gel reinforcement; remodelling; stem cells differentiation; tissue engineering; vascular patches.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- World Health Organisation Cardiovascular Diseases (CVDs) [(accessed on 8 March 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases...
-
- Yao Y., Wang J., Cui Y., Xu R., Wang Z., Zhang J., Wang K., Li Y., Zhao Q., Kong D. Effect of sustained heparin release from PCL/chitosan hybrid small-diameter vascular grafts on anti-thrombogenic property and endothelialisation. Acta Biomater. 2014;10:2739–2749. doi: 10.1016/j.actbio.2014.02.042. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

