Validation of Aspartylglucosaminidase Activity Assay for Human Serum Samples: Establishment of a Biomarker for Diagnostics and Clinical Studies
- PMID: 36982794
- PMCID: PMC10059667
- DOI: 10.3390/ijms24065722
Validation of Aspartylglucosaminidase Activity Assay for Human Serum Samples: Establishment of a Biomarker for Diagnostics and Clinical Studies
Abstract
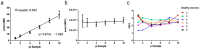
Novel treatment strategies are emerging for rare, genetic diseases, resulting in clinical trials that require adequate biomarkers for the assessment of the treatment effect. For enzyme defects, biomarkers that can be assessed from patient serum, such as enzyme activity, are highly useful, but the activity assays need to be properly validated to ensure a precise, quantitative measurement. Aspartylglucosaminuria (AGU) is a lysosomal storage disorder caused by the deficiency of the lysosomal hydrolase aspartylglucosaminidase (AGA). We have here established and validated a fluorometric AGA activity assay for human serum samples from healthy donors and AGU patients. We show that the validated AGA activity assay is suitable for the assessment of AGA activity in the serum of healthy donors and AGU patients, and it can be used for diagnostics of AGU and, potentially, for following a treatment effect.
Keywords: aspartylglucosaminuria; biomarkers; enzymes; lysosomal storage disorders; protein glycosylation.
Conflict of interest statement
A.B. declares no conflict of interest. M.L. declares consulting activities for Neurogene Inc. R.T. has received research funding from Neurogene Inc. for studies related on AGU, but not directly concerning the present study, and funding from Argenx BV, GC Pharma, and Topas Therapeutics for unrelated studies.
Figures





References
-
- Mononen I., Heisterkamp N., Kaartinen V., Williams J.C., Yates J.R., 3rd, Griffin P.R., Hood L.E., Groffen J. Aspartylglycosaminuria in the Finnish population: Identification of two point mutations in the heavy chain of glycoasparaginase. Proc. Natl. Acad. Sci. USA. 1991;88:2941–2945. doi: 10.1073/pnas.88.7.2941. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

