Natural Products and Their Derivatives as Inhibitors of the DNA Repair Enzyme Tyrosyl-DNA Phosphodiesterase 1
- PMID: 36982848
- PMCID: PMC10051138
- DOI: 10.3390/ijms24065781
Natural Products and Their Derivatives as Inhibitors of the DNA Repair Enzyme Tyrosyl-DNA Phosphodiesterase 1
Abstract
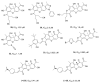
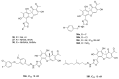
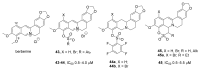
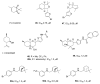
Tyrosyl-DNA phosphodiesterase 1 (TDP1) is an important repair enzyme that removes various covalent adducts from the 3' end of DNA. Particularly, covalent complexes of topoisomerase 1 (TOP1) with DNA stabilized by DNA damage or by various chemical agents are an examples of such adducts. Anticancer drugs such as the TOP1 poisons topotecan and irinotecan are responsible for the stabilization of these complexes. TDP1 neutralizes the effect of these anticancer drugs, eliminating the DNA adducts. Therefore, the inhibition of TDP1 can sensitize tumor cells to the action of TOP1 poisons. This review contains information about methods for determining the TDP1 activity, as well as describing the inhibitors of these enzyme derivatives of natural biologically active substances, such as aminoglycosides, nucleosides, polyphenolic compounds, and terpenoids. Data on the efficiency of combined inhibition of TOP1 and TDP1 in vitro and in vivo are presented.
Keywords: TDP1 inhibitors; TOP1 inhibitors; anticancer therapy; derivative; natural compound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



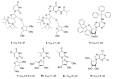
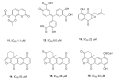
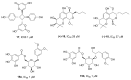
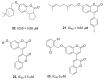

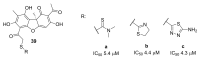





References
-
- Takashima H., Boerkoel C.F., John J., Saifi G.M., Salih M.A.M., Armstrong D., Mao Y., Quiocho F.A., Roa B.B., Nakagawa M., et al. Mutation of TDP1, Encoding a Topoisomerase I-Dependent DNA Damage Repair Enzyme, in Spinocerebellar Ataxia with Axonal Neuropathy. Nat. Genet. 2002;32:267–272. doi: 10.1038/ng987. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

