A Novel Strategy to Identify Endolysins with Lytic Activity against Methicillin-Resistant Staphylococcus aureus
- PMID: 36982851
- PMCID: PMC10059956
- DOI: 10.3390/ijms24065772
A Novel Strategy to Identify Endolysins with Lytic Activity against Methicillin-Resistant Staphylococcus aureus
Abstract
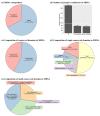
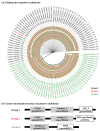
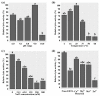
The increasing prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in the dairy industry has become a fundamental concern. Endolysins are bacteriophage-derived peptidoglycan hydrolases that induce the rapid lysis of host bacteria. Herein, we evaluated the lytic activity of endolysin candidates against S. aureus and MRSA. To identify endolysins, we used a bioinformatical strategy with the following steps: (1) retrieval of genetic information, (2) annotation, (3) selection of MRSA, (4) selection of endolysin candidates, and (5) evaluation of protein solubility. We then characterized the endolysin candidates under various conditions. Approximately 67% of S. aureus was detected as MRSA, and 114 putative endolysins were found. These 114 putative endolysins were divided into three groups based on their combinations of conserved domains. Considering protein solubility, we selected putative endolysins 117 and 177. Putative endolysin 117 was the only successfully overexpressed endolysin, and it was renamed LyJH1892. LyJH1892 showed potent lytic activity against both methicillin-susceptible S. aureus and MRSA and showed broad lytic activity against coagulase-negative staphylococci. In conclusion, this study demonstrates a rapid strategy for the development of endolysin against MRSA. This strategy could also be used to combat other antibiotic-resistant bacteria.
Keywords: Staphylococcus aureus; antibiotic resistance; bovine mastitis; endolysin; methicillin-resistant Staphylococcus aureus; prophage.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Haddad Kashani H., Schmelcher M., Sabzalipoor H., Seyed Hosseini E., Moniri R. Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: Current status of research and novel delivery strategies. Clin. Microbiol. Rev. 2018;31:e00071-7. doi: 10.1128/CMR.00071-17. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

