New Thienobenzo/Naphtho-Triazoles as Butyrylcholinesterase Inhibitors: Design, Synthesis and Computational Study
- PMID: 36982951
- PMCID: PMC10059756
- DOI: 10.3390/ijms24065879
New Thienobenzo/Naphtho-Triazoles as Butyrylcholinesterase Inhibitors: Design, Synthesis and Computational Study
Abstract
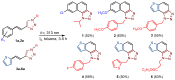
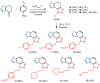
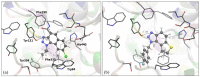
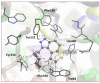
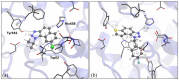
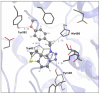
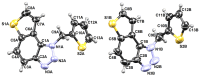
This study aims to test the inhibition potency of new thienobenzo/naphtho-triazoles toward cholinesterases, evaluate their inhibition selectivity, and interpret the obtained results by molecular modeling. The synthesis of 19 new thienobenzo/naphtho-triazoles by two different approaches resulted in a large group of molecules with different functionalities in the structure. As predicted, most prepared molecules show better inhibition of the enzyme butyrylcholinesterase (BChE), considering that the new molecules were designed according to the previous results. Interestingly, the binding affinity of BChE for even seven new compounds (1, 3, 4, 5, 6, 9, and 13) was similar to that reported for common cholinesterase inhibitors. According to computational study, the active thienobenzo- and naphtho-triazoles are accommodated by cholinesterases through H-bonds involving one of the triazole's nitrogens, π-π stacking between the aromatic moieties of the ligand and aromatic residues of the active sites of cholinesterases, as well as π-alkyl interactions. For the future design of cholinesterase inhibitors and search for therapeutics for neurological disorders, compounds with a thienobenzo/naphtho-triazole skeleton should be considered.
Keywords: 1,2,3-triazoles; cholinesterases; inhibition; photochemistry; synthesis.
Conflict of interest statement
Part of the molecules used were made in the chemical department of Selvita under the guidance and proposal by Irena Ćaleta. Ida Selec and Ana Ratković participated in the synthesis.
Figures
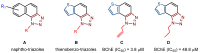

References
-
- Kovarik Z. Amino acid residues conferring specificity of cholinesterases. Period. Biol. 1999;101:7–15.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

