Long-Lasting Therapeutic Response following Treatment with Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Real-World Experience
- PMID: 36983011
- PMCID: PMC10056863
- DOI: 10.3390/ijms24065938
Long-Lasting Therapeutic Response following Treatment with Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Real-World Experience
Abstract
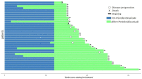
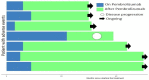
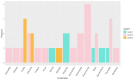
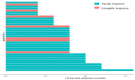
Immune checkpoint inhibitors (ICIs), pembrolizumab in particular, have been shown to be vastly more efficacious than traditional cytotoxic or platinum-based chemotherapies in the treatment of non-small cell lung cancer (NSCLC). While there are plenty of data showing their efficacy and safety profiles, very little exists about the long-term effects of pembrolizumab. We compiled all patients with NSCLC who were treated with pembrolizumab at our institution and had progression-free survival (PFS) of at least 2 years during or after the treatment period. Within this group, we examined the long-term rates of PFS and overall survival (OS), side effect profiles, treatment, and overall disease course up to 60 months after starting treatment. This study included 36 patients with median (range) follow up times from treatment initiation in months as follows: 36 (28-65) overall; 39.5 (28-65) for adenocarcinoma; and 36 (30-58) for squamous cell carcinoma. The median (range) of OS and PFS (months) was comparable for adenocarcinoma, 36 (23-55); and squamous cell carcinoma, 35.5 (28-65). Overall, pembrolizumab shows remarkable long-term safety and efficacy in NSCLC patients. In patients who show an initially strong response and can make it to 24 months of PFS, disease progression after this period seems increasingly unlikely.
Keywords: Keytruda; PD-1; immune checkpoint inhibitors; lung cancer; non-small cell lung cancer; pembrolizumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Shalata W., Yakobson A., Weissmann S., Oscar E., Iraqi M., Kian W., Peled N., Agbarya A. Crizotinib in MET Exon 14-Mutated or MET-Amplified in Advanced Disease Non-Small Cell Lung Cancer: A Retrospective, Single Institution Experience. Oncology. 2022;100:467–474. doi: 10.1159/000525188. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

